Reference
Ashar YK, Sun M, Knight K, et al. Open-label placebo injection for chronic back pain with functional neuroimaging: a randomized clinical trial. JAMA Netw Open. 2024;7(9):e2432427.
Study Objective
To test whether open-label placebo (OLP) is effective against chronic back pain (CBP) and investigate the neurobiological mechanisms responsible.
Key Takeaway
Open-label placebos administered without deception to patients are effective for chronic back pain. Changes in brain function were demonstrated via functional magnetic resonance imaging (fMRI).
Design
At a university research setting and a community orthopedic clinic, investigators conducted a randomized clinical trial of chronic back pain, using longitudinal fMRI to compare open-label placebo against usual care .
They conducted the trial from November 2017 to August 2018, with a 1-year follow-up, completing the trial in November 2019.
This study was part of a larger trial designed to facilitate 2 interventions of interest: a test of a psychotherapy intervention known as pain reprocessing therapy with OLP serving as the control condition. This article compares just OLP vs usual care and focuses on mechanistic and clinical outcomes of OLP.1
Participants
Participants were aged 21 to 70 years, and all had CBP. They were recruited from Boulder, Colorado, and had back pain for at least half the days of the past 6 months, with a 1-week average pain intensity of 4 or greater on a 10-point scale. Applicants with leg pain worse than back pain or self-reported diagnoses of inflammatory disorders or metastasizing cancers were excluded. So were people self-reporting psychosis, personality disorders, pain-related compensation, or recent litigation. Of the 101 participants who began the trial, 51 received OLP, and the remaining 50 received usual care. Forty-three of those receiving OLP and 36 of those receiving usual care completed the 12-month follow-up, and investigators included their data in the analysis.
Intervention
Active participants received a single subcutaneous lumbar injection of saline solution containing no active medication at the site of their greatest back pain. Prior to the injection, they received information about the power of placebo to relieve pain.
Study Parameters Assessed
The primary outcome was average pain over the last week on an 11-point numeric rating scale (0, no pain; 10, worst pain imaginable), as assessed with the Brief Pain Inventory–Short Form (BPI-SF).
Secondary outcomes included: pain interference (BPI-SF); Patient-Reported Outcomes Measurement Information System (PROMIS) short forms for depression, anxiety, anger, and sleep quality; Patient Global Impression of Change (PGIC); and the Treatment Satisfaction Questionnaire. Investigators collected outcomes at prerandomization and at all follow-up time points, except for the PGIC and TSQ outcomes, which cannot be measured before randomization.
Investigators obtained both structural and functional MRI images. During fMRI, participants completed an evoked back pain task with a series of randomly ordered trials distending the back to 1 of 4 intensity levels.
They also performed an 8-minute scan for each participant before and after treatment; during the scan, participants rated their spontaneous back pain intensity on a visual analog scale once per minute.
Primary Outcome
Pain intensity (on a scale of 1–10) at 1-month posttreatment was the primary outcome tracked. Secondary outcomes included pain interference, depression, anxiety, anger, and sleep quality.
Key Findings
Open-label placebo reduced chronic back pain intensity posttreatment (relative reduction, 0.61; Hedges g=0.45; 95% CI, -0.89–0.04; P=0.02).
Of the patients who received OLP, 45% reported a 30% reduction in pain, and 24% reported a 50% reduction. Together, 69% of those receiving OLP injections reported pain relief.
Pain relief was also noted in those who received only usual care: 38% reported a 30% reduction, and 15% reported a 50% reduction. Together, 53% of those receiving usual care reported pain relief.
In the OLP group, significant benefits were also observed for depression, anger, anxiety, and sleep disruption (Hedges g=0.3–0.5; all P<0.03). These benefits, along with pain relief, continued for the full year of follow-up.
Brain responses to evoked back pain for OLP vs usual care increased in the rostral anterior cingulate and ventromedial prefrontal cortex and decreased in the somatomotor cortices and thalamus. During spontaneous pain, functional connectivity analyses identified OLP vs usual care increases in ventromedial prefrontal cortex connectivity to the rostral ventral medulla, a pain-modulatory brainstem nucleus. Participants did not report any adverse effects of treatment.
“The placebo treatment also led to reduced somatomotor activity and increased medial prefrontal activity during evoked back pain and to increased medial prefrontal-brainstem functional connectivity during spontaneous pain,” investigators reported.
Transparency
Funding sources were not reported in the study.
Practice Implications & Limitations
Let’s face it: This study and the other recent findings about placebo action are hard to wrap one’s head around. While the placebo effect may be well-established, the newer findings related to open-label placebos are a mental challenge, akin to removing 1 leg from a 3-legged stool and realizing it’s still standing. Something other than belief appears to be involved in placebo action.
Most medical and naturopathic practitioners are well-aware that placebo treatments can have significant measurable effects on patient outcome. Yet, even so, many of us forget how effective placebos can be and that these treatments can have such clinically significant impact that they compete with standard therapies for effectiveness. For example, in a 2017 systematic review of the literature, Louw et al examined randomized, controlled trials that compared surgery vs sham surgery in orthopedics. They identified 6 randomized RCTS (N=277) of good methodological quality. Their evaluation suggests that the sham surgeries were as effective as actual surgery in reducing pain and improving disability.2
Another example is Enthoven et al’s 2017 review, which compared nonsteroidal anti-inflammatory drugs (NSAIDs) against placebo for chronic low-back pain. Their conclusion was that NSAIDs do work better but that conclusion lost statistical significance when “studies with high risk for bias were excluded. The associated benefits were smaller than the minimal clinically important difference.” In other words, the difference between pain relief from taking NSAIDs vs placebo wasn’t enough for patients to feel different.3
In 2012, Kailmes et al reported on the effectiveness of vertebroplasty for osteoporotic spinal fractures. In a trial (N=131), half the patients underwent vertebroplasty and half a “simulated procedure.” Both groups experienced similar significant improvements in pain and disability for the first several months following the procedure.4
In a 2020 review paper published in the British Medical Journal, Kaptchuk et al wrote that in data gathered from 140,000 patients suffering from various chronic pain conditions, placebo responses accounted for 50% to 75% of the benefits provided by drug treatments for pain. Similar percentages are seen for relief of cancer-related fatigue and menopausal hot flashes.5
Even if poorly understood, the placebo effect is well-documented, and there is little doubt that well-administered placebos relieve pain and discomfort, but that is not the issue here.
In the United Kingdom, placebo use was even higher: In a 2013 survey, 97% of primary care doctors admitted to using a placebo at least once in their career, and 77% said they prescribed a placebo at least once a week.
There is an important problem with prescribing placebos. In the last half-century, intentional placebo use has become ethically questionable. Concepts of patient autonomy that arose in the mid-1900s have brought emphasis to the idea that physicians should be forthcoming and provide patients adequate and truthful information about their diagnoses, prognoses, and treatment. Placebos, because they require deception, threaten a patient’s autonomy and ability to make informed decisions. As physicians, we are expected to be honest in all our communications with patients.
How could a health practitioner prescribe a placebo without practicing deception? Although there are papers in the medical journals that debate the difference between deception and lying, it is hard to get around the idea that placebo use requires us to do one or both. Our modern sensibility insists that doctors should be honest practitioners. The problem is that giving a placebo is inherently a dishonest act, and for doctors and scientists, deceiving patients is ethically frowned upon.6
Such ethical worries don’t stop doctors from using placebos. In 2008 more than half of internists and rheumatologists in the US admitted to using placebos—that is, “a medication such as vitamins or analgesics that would have no effect on the illness but were prescribed for their psychological value.”7 In the United Kingdom, placebo use was even higher: In a 2013 survey, 97% of primary care doctors admitted to using a placebo at least once in their career, and 77% said they prescribed a placebo at least once a week.8
It was in 2010 that Theodore Kaptchuk turned our concept of what a placebo is upside down by doing something bizarre: He conducted a clinical trial with “open-label placebos.” Both the doctors prescribing the placebo and the patients receiving the placebo knew and were made well-aware that the intervention was a placebo. No deceit was involved. Patients with diagnosed irritable bowel syndrome (IBS; N=80) were divided into 2 groups, half of whom received inert sugar pills and the other half nothing. Those who received the sugar pill experienced global symptom improvement by day 11, and by the end of the trial, at day 21, they had experienced significant reductions in symptom severity and relief of symptoms. Even their quality of life was trending toward improvement.9 In 2022, in a similar study, improvements were also seen in children with IBS symptoms (N=30).10
Kaptchuk summarized his ongoing research in The New York Times in October 2023, stating, “Currently, more than a dozen randomized trials demonstrate that open-placebo treatment can reduce symptoms in many illnesses with primarily self-reported symptoms such as chronic low back pain, migraine, knee pain and more. These findings suggest that patients do not have to believe, expect or have faith in placebos to elicit placebo effects.”11
If Kaptchuk’s name seems familiar, it is because he wrote a well-known book on acupuncture, The Web That Has No Weaver, first published in 1983, which has been a frequently used textbook in naturopathic schools.
Kaptchuk’s “honest” placebos require no deception to work and eliminate concerns of crossing an ethical line by using deception.12
The benefits seen in Ashar et al’s current trial are nearly identical to those reported in another chronic back pain study published in 2019. In that study, Kleine-Borgmann reported modest, but significant, pain reductions using OLP for CBP (pain reduction of 0.61 on an 11-point scale). Common CBP treatments, such as NSAIDS and epidural steroid injections, have about the same impact but come with the risk of adverse events. In 2016, Carvalho et al, in a similar study of OLP for CBP, reported larger pain reductions, suggesting that OLP effects may be magnified in certain contexts.13
One quite notable aspect to the results reported in this Ashar back pain study is the persistence of the improvements. Reductions in depression, anxiety, and anger and improvements in sleep persisted through a full 1-year of follow-up. These improvements had not reached significance at the 1-month follow-up but accrued gradually over time. Such gradual changes might be explained by saying improvements in any 1 of these traits spill over and create improvements in these other parameters.14 Considering that the OLP saline injections were given just 1 time, we must either doubt the reported results or simply marvel at the duration of the phenomenon triggered.
It is as if some switch were thrown somewhere in the brain that turns on a homeostatic system designed to restore a healthy equilibrium, kind of like when a popup screen appears on your computer offering to restore the computer’s original system setting and you click it.
In this study, Ashar et al offer a potential rationale for what seem like global changes in neuropsychological processes. This reader found some of the data garnered from fMRI studies to be over his head (pun sort of intended), especially the conceptual frameworks that explain the mind-brain processes. Analogies and metaphors that come to mind may or may not reflect the reality or complexity of what is happening. It does seem that if we might understand these neuropsychological processes, we might be able to better mediate placebo effects and enhance a broad range of therapeutic interventions.15
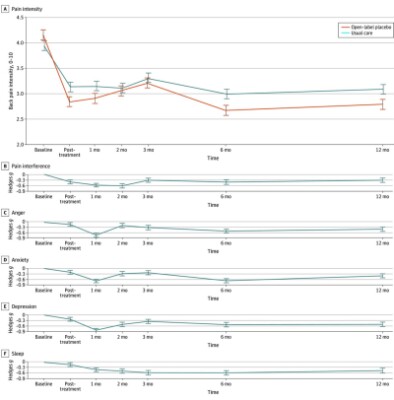
Fig 1. Copyright 2024 Ashar YK et al. JAMA Network Open. This is an open access article distributed under the terms of the CC-BY License. Original images available here.
As naturopathic doctors, we are aware of the power and the challenge placebos bring to a case. Samuel Hahnemann, who developed the system of homeopathy that many of us incorporate into our practices, was 1 of the first medical practitioners to purposefully prescribe placebos. In fact, according to Robert Jütte, he was the first physician to administer placebos to his patients in a systematic and regular manner. According to his practice guidelines, published between 1810 and 1830, Hahnemann suggests that homeopathic practitioners prescribe “placebo as a wash-out when discontinuing allopathic medication, and at the beginning of homeopathic treatment, to identify ‘placebo responders’. It [placebo] was also frequently used in longer-term case management, because the single rarely-repeated doses of active medicines used in homeopathy were believed to produce misleading psychosomatic responses.”16,17
Examination of Hahnemann’s case journals suggests that the majority (54% to 85%) of his prescriptions were for placebos, with only occasional prescriptions for active remedies spaced between them. The justification for this was that patients at that time were accustomed to taking medicines daily or several times during the day. Patients expected such dosing, and the placebos were used to humor them.18
Homeopaths also used placebo controls in early therapeutic trials and “provings,” and this use was the origin of the single-blind placebo controls used today in controlled trials.19 Some sources tell us that homeopathy was subjected to large, placebo-controlled trials starting in the 1830s,20 but actually, there are earlier such studies. Two hospital-based studies were conducted by the Russian military, starting in 1829. The first of these took place in Podolya, Ukraine. Patients in 1 hospital ward (N=164) were treated with homeopathy and were compared with patients in a second ward who were treated with standard care. Results of the homeopathic patients were favorable and intriguing enough that a second experiment was set up in St Petersburg. This second trial was different in that a third arm was added to the study, and in that arm, 1 ward’s patients received only placebo and palliative care. Recall that active medical care at that time consisted of bleeding and other heroic interventions. The group receiving palliative care and placebo apparently had the best results.17
The Ashar study has more and more company. Multiple studies now document OLP effects. A 2017 meta-analysis by Charlesworth et al found 5 trials (N=260) that met inclusion criteria. The clinical conditions included irritable bowel syndrome, depression, allergic rhinitis, back pain, and attention deficit hyperactivity disorder. The risk of bias was moderate. They found a positive effect for OLPs (standardized mean difference=0.88, 95% CI 0.62–1.14, P<0.00001, I2=1%).21
Additionally, 2021 review by von Wernsdorff et al included a meta-analysis of 11 trials and found a significant overall effect (standardized mean difference=0.72, 95% Cl 0.39–1.05, P<0.0001, I2=76%) of OLP.22
In 3 more recent studies: In an August 2024 meta-analysis of OLP use in allergic rhinitis, Albazee et al reported benefit in reducing frequency of symptoms23; in September 2024, Schaefer et al reported that OLP aided weight loss in a randomized, controlled study of obese individuals (N=57);24 and in a fascinating study from May 2024, Schienle and Kogler reported that taking an imaginary pill was superior in reducing anxiety compared to taking an actual OLP pill.25
The fact that human health may be so influenced by placebo and open-label placebo obviously raises a great many questions about the practice of medicine. People will often feel improvement after medical interventions. It is a challenge to determine how much of the improvement is the result of the medicine or the placebo action. Ideally, we would like as much improvement as possible and would prefer if both the medicine and the placebo effect were congruent if not synergistic. The medical community has viewed randomized, placebo-controlled trials as a solution for deciphering what is an acting agent by comparing results between those receiving an active intervention and those receiving placebo. Whether we can remove the deceptive aspect of these studies and compare active medicines against open-label placebos is a question we are unable to answer quite yet.
Even when using medicines that we are fairly certain produce beneficial actions greater than what a placebo will provide, we must assume that some placebo effect is involved.
That Ashar et al were able to identify discernable effects of placebo on brain activity via fMRI is intriguing as it suggests that researchers may eventually be able to determine how to maximize placebo effect in patients via qualitative measurement of brain activity.
At this point, how might this information inform our patient interactions? I suspect some practitioners purposefully express greater confidence in their suggested interventions than justified by current research in the hope it will bolster placebo action. Ashar et al’s study and other OLP studies would suggest this is unnecessary. It may be that being truthful and saying, “We are not certain that this will work. It may only be a placebo,” when describing a proposed intervention may not lessen its power to trigger benefit. Nor do we need to omit mention of negative studies. We might say, “Research on whether this is effective varies. Some studies suggest it does, while others are unable to confirm the benefit.” Being truthful whenever possible seems to be a superior policy.
Conflict-of-Interest Disclosure
The author declares no conflict of interest.






