Diminished ovarian reserve, a natural process by which the follicular pool diminishes with time, is often clinically asymptomatic but presents a challenge to those wanting to become pregnant. Although pelvic ultrasonography and day 3 serum hormone testing have long been common methods of assessing ovarian reserve, promising research suggests the utility of anti-mullerian hormone testing to identify patients with diminished ovarian reserve. Conventional wisdom would suggest that in vitro fertilization is the only answer for diminished ovarian reserve, but preliminary research offers hope that egg quality and ovarian reserve can be improved by dehydroepiandrosterone, melatonin, and myo-inositol.
Abstract
Diminished ovarian reserve, a natural process by which the follicular pool diminishes with time, is often clinically asymptomatic but presents a challenge to those wanting to become pregnant. Although pelvic ultrasonography and day 3 serum hormone testing have long been common methods of assessing ovarian reserve, promising research suggests the utility of anti-mullerian hormone testing to identify patients with diminished ovarian reserve. Conventional wisdom would suggest that in vitro fertilization is the only answer for diminished ovarian reserve, but preliminary research offers hope that egg quality and ovarian reserve can be improved by dehydroepiandrosterone, melatonin, and myo-inositol.
Introduction
Infertility is often initially evaluated in the primary care setting after 12 months of regular, unprotected intercourse in women younger than age 35, and after 6 months of regular intercourse in women older than age 35.1 Evaluations can be initiated earlier in women with irregular cycles, suspected anovulatory cycles, or other known risk factors for infertility, such as endometriosis, polycystic ovaries, or a history of pelvic inflammatory disease. Both partners should undergo a complete evaluation concurrently, since multiple factors can affect fertility. Poor semen parameters, anovulation, tubal disease, poor egg quality, and endocrine imbalances are some of the causes of infertility, each with its own treatment approaches.
Diminished ovarian reserve (DOR) is a natural phenomenon in which the number and quality of a woman’s eggs diminish as she ages in a process called atresia. The loss of the primordial egg pool begins at 20 weeks of gestation, and by menarche, the primordial egg pool contains an estimated 500,000 follicles.2 As the follicular pool wanes with time, female fecundity (ie, ability to reproduce) diminishes. A woman is said to have a diminished ovarian reserve when her follicular pool is less than what is expected for her age. Whether due to the physiologic aging of the ovaries or premature ovarian aging, diminished ovarian reserve is often clinically asymptomatic and represents one of the most challenging infertility diagnoses to overcome.
Evaluating Ovarian Reserve
Reproductive endocrinologists assess ovarian reserve through a number of diagnostic tests. One such test is the pelvic ultrasound performed early in the menstrual cycle to evaluate the number of follicles present. The more follicles present (known as the “antral follicle count”), the greater that patient’s ovarian reserve. Pelvic ultrasonography, although a useful diagnostic tool, is highly user-dependent and is not available for use in primary care and naturopathic medical clinics. Another means of assessing ovarian reserve is through serum hormone testing. Historically, the most common serum tests to assess egg quality have been follicle stimulating hormone (FSH) and estradiol on day 3 of the menstrual cycle. FSH is a measure of how much stimulation the ovaries require to function. An FSH measurement greater than 10–12 mIU/mL has been identified as the upper limit of normal,3 whereas estradiol greater than 80 pg/mL is considered the upper limit of normal.4 Estradiol is measured concomitantly with FSH in part to determine the validity of FSH testing. If estradiol is elevated, FSH is suppressed, and this can appear as a falsely normal or low FSH level. The accuracy of day 3 serum evaluation is affected by endogenous hormone production (such as a functional ovarian cyst producing estrogen), exogenous hormones (such as infertility medications), and the timing of the sample collection.5
Overall, AMH demonstrated the strongest inverse correlation with the number of retrieved oocytes.
In the last several years, anti-mullerian hormone (AMH) testing has been studied for use in the assessment of egg quantity. AMH is a peptide hormone from the TGF-β family made from birth to menopause by the functional granulosa cells of the preantral follicles. Therefore as the preantral follicle count diminishes with time, AMH levels diminish as well.6,7 In addition, serum studies have demonstrated that AMH levels are stable throughout the menstrual cycle and demonstrate minimal variability between menstrual cycles.8–11 A recent retrospective study in the American Journal of Obstetrics and Gynecology compared the number of oocytes retrieved during a given round of in vitro fertilization (IVF) with AMH, inhibin B, day 3 estradiol, and day 3 FSH.12 No correlation was seen between the number of oocytes retrieved and inhibin B, but AMH, FSH, and estradiol demonstrated statistically significant inverse correlation with the number of oocytes retrieved. Overall, AMH demonstrated the strongest inverse correlation with the number of retrieved oocytes.12 Riggs et al established a sensitivity of 83–84% and specificity of 67–79% using ROC curve analysis data.13 In addition, Barad et al, in a study investigating the utility of age-specific AMH, determined that AMH provides the best specificity for diminished ovarian reserve in women ages 32–39;14 however, the same team concluded that AMH testing is particularly beneficial for younger women because diminished ovarian reserve is often unsuspected in younger women, leading to the inaccurate diagnosis of “unexplained infertility.” A growing number of retrospective and prospective data corroborate the utility of AMH as a marker for ovarian reserve.15–24
Despite its value in assessing ovarian reserve, AMH testing has several shortcomings. First, there is no uniform reference range for interpreting AMH levels in regard to ovarian reserve, largely because different laboratories use different assays (see Table 1 and Figure 1). Gnoth et al determined an AMH of less than 1.26 ng/ml denotes a diminished ovarian reserve regardless of age.25 Ebner et al determined that an AMH between 1.7 and 4.5 ng/ml results in maximal egg quality.26 Tremellen et al defined diminished ovarian reserve as an AMH less than 0.8 ng/ml, which they specify is comparable to an FSH of 11 mIU/ml.27 Second, despite an emergence of promising research on the correlation of AMH with ovarian reserve and egg quality, there is conflicting data regarding the correlation of AMH with pregnancy rates.21,28,29 Notwithstanding, a study conducted by Barad et al demonstrates that AMH is superior to FSH in predicting IVF outcomes.30 And lastly, there is now some emerging research demonstrating that AMH levels can be influenced by race, body mass index, and polycystic ovaries.31,32,33 Some preliminary research has shown that women with polycystic ovaries have AMH levels 2- to 3-fold higher than women with ovulatory cycles.34 AMH studies controlling for these variations are pending. Given the emerging data demonstrating the utility and convenience of AMH testing, it should be routinely considered in the diagnostic evaluation of all infertile women to determine ovarian reserve.
Treatments
There is very little research demonstrating strategies to improve ovarian reserve. Conventional wisdom would question whether or not that is even plausible. However, some preliminary data on the use of dehydroepiandrosterone, melatonin, and myo-inositol offers hope for women with diminished ovarian reserve.
Dehydroepiandrosterone (DHEA)
The first study demonstrating potential improvement in ovarian reserve with dehydroepiandrosterone (DHEA) supplementation was published more than a decade ago. Casson et al presented data documenting the positive effect of DHEA supplementation on oocyte yields in patients with a prior history of poor response during IVF cycles.35 The effect was caused by increasing IGF-1 and augmenting the effects of gonadotropin medications. In an attempt to further elucidate the mechanism by which DHEA improved egg quality, Sen and Hammes conducted a mouse study showing that DHEA increases androgens that regulate ovarian development and function by promoting preantral follicular growth and preventing follicular atresia.36 Initial attempts to reproduce human studies in patients with DOR have proven challenging due to the fact that many infertility patients decline randomization during the initial trial phase, due to concerns about wasting precious time. Barad and Gleicher are conducting much of the latest research on DHEA use during IVF; they recommend compounded micronized DHEA at a dose of 25 mg tid for at least 6–8 weeks before initiating an IVF cycle, with effects plateauing after 5 months of supplementation.37–39 Instead of a randomized controlled study, Barad and his team documented the effects of pre- and post-DHEA IVF cycles. In 25 women diagnosed with DOR, DHEA supplementation improved the numbers of oocytes retrieved, fertilized oocytes, day 3 blastomeres, transferred embryos, and normal day 3 embryos.40 In a follow-up study, Barad found that when 89 DOR patients supplemented with DHEA for up to 4 months, they had a shorter time to pregnancy, as well as higher pregnancy rates when compared to 101 controls (pregnancy rate of 28.1% in DHEA-supplemented women vs 10.9% in controls).39 When age-dependent DOR patients and patients with premature ovarian aging are separated and compared, it appears as though DHEA is similarly effective in both groups.39 The latest study, conducted by Gleicher et al, demonstrated that women taking DHEA had improvement in AMH, which also correlated with increased pregnancy rates.38 Gleicher also suggests that DHEA may reduce aneuploidy in oocytes and embryos,41 thus improving pregnancy rates. Wiser et al have demonstrated similar effects of DHEA supplementation, also using a dose of 25 mg tid.42 Recently and most surprisingly, Gleicher and Barad documented a spontaneous pregnancy in a 38-year-old patient with premature ovarian failure after 4 months of DHEA supplementation, her highest FSH previously being 100.0 mIU/mL. They are now investigating DHEA supplementation in patients with premature ovarian failure, with results to be published after 2013.14 Barad and Gleichner have also documented that women taking DHEA complain of very few side effects; the most common adverse effects reported with DHEA use are oily skin, acne vulgaris, and mild hair loss.38 Other studies have corroborated that hirsutism and acne are the most commonly reported side effects of similarly dosed DHEA, while oral stomatitis, myalgias, headaches, irritability, and nausea are less common complaints.43
Melatonin
Melatonin has been studied as an antioxidant, free-radical scavenger, and nutrient that can modulate gene transcription for antioxidant enzymes.44–47 There is some evidence that reactive oxygen species are made by the follicles during ovulation and that there is less glutathione reductase in the follicular fluid of infertile women.48,49 In an effort to investigate the relationship between oxidative stress and egg quality, Tamura et al studied the effect of melatonin supplementation in infertile women with poor egg quality. Women who had failed their first round of IVF were divided into 2 groups before initiating their second round of IVF; 56 women received melatonin supplementation (3 mg per day) and 59 women received no melatonin. Women who received melatonin supplementation had slightly improved pregnancy rates, although this trend was not statistically significant.50 Recently a placebo-controlled study conducted by Batioglu et al compared the effects of administering 3 mg/day of melatonin (n=40) versus no treatment (n=45) to age-matched women undergoing IVF. Women who received melatonin supplementation had modest improvement in the percentage of mature oocytes retrieved, embryo quality, and clinical pregnancy rate. Although the difference was not statistically significant, Batioglu et al speculate that melatonin has a positive impact on oocyte and embryo quality.51 More research on melatonin use for DOR is warranted to ascertain if it is an effective treatment option to improve ovarian reserve.
Myo-inositol
Myo-inositol, an isomer of inositol made from glucose-6-phosphate, has been said to improve insulin sensitivity and oocyte maturation.52 Myo-inositol has been shown to be an important constituent in the follicular microenvironment wherein higher levels of myo-inositol in the follicular fluid have been correlated to improved egg quality.53,54 Papaleo et al studied 60 infertile women undergoing IVF; they were given 2 grams of myo-inositol twice daily with 400 mcg folic acid (n=30) or 400 mcg folic acid alone (n=30). Cycles were canceled when E2 levels exceeded 4,000 pg/ml as this indicates a higher risk for ovarian hyperstimulation syndrome. Women in the group receiving myo-inositol required a lower dose of ovarian stimulation medication, had lower peak E2 levels, and had a decreased mean number of immature oocytes. Only 1 cycle had to be canceled in the group receiving myo-inositol, whereas 3 cycles were canceled in the control group due to elevated E2 levels. Although there was no statistically significant difference in the pregnancy rate between the myo-inositol group (11 pregnancies) and the control group (10 pregnancies), their findings suggest myo-inositol supplementation has a positive impact on oocyte development and maturation.55 A similar double-blind study was conducted in Italy in which women diagnosed with polycystic ovaries were given 2 grams of myo-inositol with 200 mcg folic acid twice daily or 200 mcg folic acid alone twice daily for 3 months. During ovarian stimulation protocols, women who had taken the myo-inositol had more follicles measuring greater than 15 mm on ultrasonography, a greater number of oocytes recovered at retrieval, and a greater number of embryos suitable for transfer, suggesting that myo-inositol can influence ovarian reserve and egg quality.56
Conclusion
Given the aforementioned data on natural therapies for improving ovarian reserve, DHEA has the most compelling positive research demonstrating improvement in ovarian reserve. Although melatonin and myo-inositol demonstrated some improvement, the effects were not statistically significant and therefore these supplements should only be offered if a patient is not tolerating side effects of DHEA supplementation, until further research is conducted.
Table 1
Average hormone concentrations among 792 women demonstrating baseline FSH<12 mIU/ml and estradiol < 80 pg/ml. A DSL-10-14400 active Mullerian inhibiting substance/anti-Mullerian hormone ELISA was used (Diagnostic Systems Laboratories; Webster, USA); it used an enzymatically amplified 2-site immunoassay that does not cross-react with other proteins from the TGF-beta family. (Adapted from Barad 2011)
| Age(years) | AMH (ng/ml) | FSH (mlU/ml) | Estradiol (pg/ml) |
| 27 | 2.7 | 6.8 | 46.2 |
| 33 | 1.9 | 7.1 | 47.8 |
| 36 | 1.34 | 7.6 | 52.2 |
| 39 | 0.98 | 7.8 | 47.5 |
| 42 | 0.56 | 8.0 | 57.1 |
| | | | |
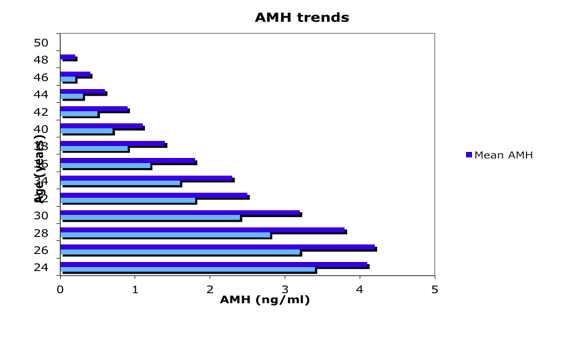
Figure 1
Median and mean AMH levels in 17,120 women ages 24–50 years presenting at U.S. IVF centers. An AMH assay from Beckman Coulter-DSL was used at a single reference laboratory called Repro-Source Inc. in Woburn, MA. (Adapted from Seifer 2011)24
Table 2
Summary of Natural Treatments to Improve Ovarian Reserve
| Dehydroepiandrosterone | 25 mg TID | |
| Melatonin | 3 mg QD | |
| Myo-inositol | 2000 mg BID | |
1. Reproductive Endocrinology and Infertility Committee, et al. Advanced reproductive age and fertility. J Obstet Gynaecol Can. 2011;33(11):1165-75.
2. Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342-46.
3. Barad DH, Weghofer A, Gleicher N. Age specific levels for basal follicle-stimulating hormone assessment of ovarian function. Obstet Gynecol. 2007;109:1404-10.
4. Smotrich DB, Wildra EA, Gindoff PR, Levy MJ, Hall JL, Stillman RJ. Prognostic value of day 3 estradiol on in vitro fertilization outcome. Fertil Steril. 1995;64(6):1136.
5. Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-Mullerian hormone: a new marker for ovarian function. Reproduction. 2006;131:1-9.
6. Ibid
7. Weenan C, Laven JS, Von Bergh AR, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77-83.
8. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057-63.
9. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Human Reprod. 2006;21:3103-7.
10. Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy Ch, Englert Y. Stable serum levels of anti-Mullerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Human Reprod. 2007:22:1837-40.
11. Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggest multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Human Reprod. 2005;20:923-7.
12. Riggs RM, Duran EH, Baker MW, et al. Assessment of ovarian reserve with anti-Mullerian hormone: a comparison of the predictive value of anti-Mullerian, follicle-stimulating hormone, inhibin B, and age. Am J Obstet Gynecol. 2008;199:202.e1-202.e8
13. Ibid
14. Barad DH, Weghofer A, Gleicher N. Utility of age-specific serum anti-Mullerian hormone concentrations. Reprod BioMed. 2011;22:284-91.
15. Seifer DB, MacLaughlin DT, Christian BP, Feng B, Sheldon RM. Early follicular serum mullerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468-71.
16. de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC, Anti-Mullerian hormone serum levels: a putative marker for ovarian aging. Fertl Steril. 2002;77:357-62.
17. van Rooij, Broekmans FJ, te Velde ER, et al. Serum anti-Mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065-71.
18. Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-Mullerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45:20-4.
19. Eldar-Geva T, Ben-Chetrit A, Spitz IM, et al. Dynamic assays of inhibin B, anti-Mullerian hormone and estradiol following FSH stimulation and ovarian ultrasonography as predictors of IVF outcome. Human Reprod. 2005;20:3178-83.
20. Muttukrishna S, McGarrigle H, Wakim R, Khadum I, Ranieri DM, Serhal P. Antral follicle count, anti-Mullerian hormone and inhibin B: predictors of ovarian response in assisted reproductive technology? BJOG. 2005;112:1384-90.
21. Kwee J, Schats R, McDonnell J, Themmen A, de Jong F, Lambalk C. Evaluation of anti-Mullerian hormone as test for the prediction of ovarian reserve. Fertil Steril. 2007.
22. Singer T, Barad DH, Weghofer A, Gleicher N. Correlation of anti-Mullerian hormone and baseline follicle-stimulating hormone levels. Fertil Steril. 2009;91:2616-19.
23. La Marca A, Volpe A. Anti-Mullerian hormone in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol. 2006;64:603-10.
24. Seifer DB, Baker VL, Leader B. Age-specific serum anti-Mullerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95:747-50.
25. Gnoth C, Schuring AN, Friol K, Tigges J, Mallmann P, Godehardt E. Relevance of anti-Mullerian hormone measurement in a routine IVF program. Hum Reprod. 2008; 23:1359-65.
26. Ebner T, Sommergruber M, Moser M Shebl O, Schreier-Lechner E, Tews G. Basal level of anti-Mullerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022-26.
27. Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-Mullerian hormones as marker of ovarian reserve. J Obstet Gynceol. 2005;45:20-24.
28. Nelson SM, Yates RW, Fleming R. Serum anti-Mullerian hormone and FSH: prediction of live birth and extreme response in stimulated cycles – implications for individualization of therapy. Hum Reprod. 2007;22:2414-21.
29. Penarrubia J, Fabregues F, Manau D, et al. Basal and stimulation day 5 anti-Mullerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist-gonadotropin treatment. Hum Reprod. 2005;20:915-22.
30. Barad DH, Weghofer A, Gleicher N. Comparing anti-Mullerian hormone and follicle-stimulating hormone as predictors of ovarian function. Fertil Steril. 2009;91:1553-55.
31. Seifer DB, Golub ET, Lambert-Messerlian G, Benning L, Anastos K, Watts DH, et al. Variations in serum mullerian inhibiting substance between white, black and Hispanic women. Fertl Steril. 2009;92:1674-8.
32. Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss 3rd JF. Association of anti-Mullerian hormone levels with obesity in the late reproductive-age women. Fertil Steril. 2007;87:101-6.
33. Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D. Anti-Mullerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab. 2009;296:E238-43.
34. Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BC. Anti-Mullerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endrincol Metab. 2004;89(1):318.
35.Casson PR, Lindsay MS, Pisarka MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Human Reprod. 2000;15:2129-32.
36. Sen A, Hammes SR. Granulosa cell-specific androgens receptors are critical regulators of development and function. Mol Endocrinol. 2010.24:1393-403.
37. Gliecher N, Barad D. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR). Reprod Bio Endocrin. 2011;9:67.
38. Gleicher N, Weghofer A, Barad DH. Improvement in diminished ovarian reserve after dehydroepiandrosterone supplementation. Reprod Biomed Online. 2010;21:360-65.
39. Barad D, Gliecher N. Update on the use of dehydroepiandrosterone supplementation among women with diminished ovarian reserve. J Assist Reprod Genet. 2007;24:629-34.
40. Barad D, Gliecher N. Effect of dehydroepiandrosterone on oocytes and embryo yields, embryo grade and cell number in IVF. Hum Reprod. 2006; 21:2845-49.
41. Gleicher N, Ryan E, Weghofer A, Bianco-Mejia S, Barad D. Dehydroepiandrosterone reduces miscarriage rates in women with diminished ovarian reserve: a multicenter study. Reprod Biol Endocrinol. 2009;7:108.
42. Wiser A, Gonen O, Ghetler Y, Shavit T, Berkovitz A, Shulman A. Addition of dehydroepiandrosterone (DHEA) for poor responder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study. Hum Reprod. 2010;25:2496-500.
43. Petri MA, Mease PJ, Merril JT, et al. Effects of prasterone on disease activity and symptoms in women with active systemic lupus erythematosus. Arthritis Rheum. 2004; 50(9):2858-68.
44. Tam DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J. 1993;1:57-60.
45. Tan DX, Manchester LC, Terron MP, Flores MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42:28-42.
46. Zavodnik IB, Domanski AV, Lapshina EA, Bryszewska M, Reiter RJ. Melatonin directly scavenges free radicals generated in red blood cells and a cell free system: chemiluminence measurements and theoretical calculations. Life Sci. 2006;79;391-400.
47. Tomas-Zapico C, Coto-Montes A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J Pineal Res. 2005;39:99-104.
48. Sugino N. Reactive oxygen species in ovarian physiology. Reprod Med Biol. 2005;4:31-44.
49. Paszkowski T, Traub AI, Robinson SY, McMaster D. Selenium dependent glutathione peroxidase activity in human follicular fluid. Clin Chim Acta. 1995;236:173-80.
50. Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44:280-87.
51. Batioglu AS, Sahin U, Ozturk N, Unsal E. The efficacy of melatonin administration on oocyte quality. Gynecol Endocrinol. 2011. [Epub ahead of print] Accessed on 1-8-12.
52 Unfer V, Carlomagno G, Dante G, Facchinetti F. Effects of myo-inositol in women with PCOS: a systemic review of randomized controlled trials. Gynecol Endocrinol. 2012. [Epub ahead of print].
53. Chiu TT, Rogers MS, Law ELK, Briton-Jones CM, Cheung LP, Haines CJ. Follicular fluid and serum concentrations of myo-inositol in patients undergoing IVF: relationship with oocyte quality. Hum Reprod. 2002;17:1591-6.
54. Chiu TT, Rogers MS, Briton-Jones C, Haines C. Effects of myo-inositol on the in vitro maturation and subsequent development of mouse oocytes. Hum Reprod. 2003;18: 408-16.
55. Papaleo E, Unfer V, Baillargeon JP, Fusi F, Occhi F, Lucia DS. Myo-inositol may improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Fertil Steril. 2009;91:1750-54.
56. Ciotta L, Stracquadanio M, Pagano I, Carbonaro A, Palumbo M, Gulino F. Effects of myo-inositol supplementation on oocyte’s quality in PCOS patients: a double blind trial. Eur Rev Med Pharmacol Sci. 2011;5:509-14.