Abstract
Atherosclerotic cardiovascular disease resulting in a heart attack is a major cause of morbidity and mortality, claiming millions of lives every year and killing more people than all wars combined.1 Traditional risk factors are largely inadequate for real risk assessment especially in intermediate-risk individuals. Both coronary calcium scoring and carotid intima thickness are approved imaging tests for further stratifying risk in the intermediate-risk patient. Clinicians can stratify individual risk more precisely using these imaging technologies, allowing for more targeted approaches to treatment.
Introduction
Eighty million adults (1 in 3 people) are affected by heart disease, and 25% of deaths in the United States are due to heart disease. From 1999 to 2009, the cardiovascular disease death rate declined by 33%. However, cardiovascular disease still takes the lives of more than 2,150 Americans each day, an average of 1 death every 40 seconds,2 and it is still the No. 1 cause of death. In 2010, coronary artery disease alone was projected to cost the United States $108.9 billion.3 Most heart attacks occur because of atherosclerotic plaque, but unfortunately, atherosclerosis remains asymptomatic, delaying treatment until it is too late.4
The number-one killer* is still largely preventable. One reason heart attacks and stroke are still so prevalent is due to the lack of preventive cardiology training given to healthcare providers worldwide (ie, lack of investment). Preventive cardiology may be described as the aggressive early detection and treatment of cardiovascular conditions such as coronary artery disease and hypertension. Since most atherosclerosis is asymptomatic, imaging allows us to detect it in early, preclinical stages, allowing for early intervention. The purpose of this article is to review the imaging that is most clinically useful to the practitioner, providing scientific support and rationale for its use. Only imaging technologies that are the most clinically useful will be addressed.† The prevention of heart attacks and stroke is something any medical practitioner can excel at.
The problem: The current standard of care does not include detection of asymptomatic atherosclerosis. Instead, traditional risk factors are evaluated, the patient is put into a low-, medium-, or high-risk category, and lipid targets are determined by the clinician depending on the category. Thus, coronary artery disease is not detected early. We know that early detection of cancer saves lives. The same is true for atherosclerosis.
A one-size-fits-all approach based only on guidelines leaves some patients over-treated and others painfully under-treated.5 A report based on guidelines initiated by the American Heart Association examining 136,905 patients hospitalized with the diagnosis of coronary artery disease (CAD) revealed the inadequacy of low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides in identifying high-risk patients. The report showed that 77%, 45%, and 62% of the patients had normal LDL, HDL, and triglycerides, respectively. This study confirmed prior suspicions suggesting poor prediction using traditional risk factors (in particular lipids), thus highlighting the shortcomings of existing national cholesterol education program guidelines.6 This should not be surprising as atherosclerosis is primarily an inflammatory condition and cholesterol is not the sole cause. There are many risk factors.
The issue of what to do about high cholesterol is a daily issue for most practitioners. Does the patient
have atherosclerosis or just high cholesterol? What should the treatment be? Does the patient have the type of plaque that could rupture? What is the real 10-year risk of having a coronary event for the patient sitting in front of you?
Determining risk: The Adult Treatment Panel III (ATP III) of the National Cholesterol Education Program (NCEP)7 algorithm, which is the most widely used system, categorizes individuals into 3 risk categories. The national lipid association uses f categories.
Framingham scoring/What are the traditional risk factors?
- Cigarette smoking
- Hypertension
- High total cholesterol
- Low HDL
- Family history of premature coronary heart disease
- Age ≥45 years in men
- ≥55 years in women
- Points are given or taken away for each category. For instance, for an HDL above 60, subtract 1 point; for an HDL below 40, ad 2 points. Most practitioners use the online calculator.8
National Cholesterol Education Program (NCEP) Risk Categories
| Low risk | 0-1 risk factors (10-year risk <10% using Framingham scoring) | Goal LDL: no higher than 160 mg/dL |
| Intermediate risk | Multiple (2+) traditional risk factors (10-year risk <20% using Framingham scoring) | Goal LDL: no higher than 130 mg/dL |
| High risk | Existing heart disease, diabetes or a 10-year risk of CHD events ≥20% using Framingham scoring | Goal LDL: no higher than 100 mg/dL |
The concept of risk factors is important but risk factors alone do not tell us if a patient is going to have a heart attack or tell us how much plaque there is. Only an image can do that. Traditional risk factors do a poor job of determining risk, especially for those in the intermediate risk range. Traditional risk factors may identify persons at very low or very high risk of heart attack or stroke in the next 10 years, but the majority of the population belongs to an intermediate-risk group for which the predictive power of risk factors is low.9 This leaves some patients over-treated and others under-treated.
Are you over-treating or under-treating a particular patient? In the case of under-treatment, take the example of a 40-year-old non-hypertensive male who is sedentary, slightly overweight, and has a first degree relative who had a heart attack before age 55. His systolic blood pressure is 140, total cholesterol is 220, triglycerides are 150, and HDL is 40. Using the Framingham risk factor criteria, his 10-year risk of a cardiovascular event would be low or 2% (2/100 people). Current recommendations for this patient include lowering LDL to a target of 130 mg/dL and recommending regular exercise and a prudent nutrient-dense diet.
One reason heart attacks and stroke are still so prevalent is due to the lack of preventive cardiology training given to healthcare providers worldwide.
Now, this patient tells you he had a heart scan last week and that he has plaque in multiple coronary arteries. He is in the 80th percentile for his age and gender for coronary artery calcium (calcium scoring). His calcium volume is 38. He is now in a high-risk category because he has been found to have CAD in addition to his other risk factors. Targeting LDL at 130 mg/dL will probably fall short of the treatment goal because he is now classified as high-risk. His LDL should now be at or below 70 mg/dL.
Who has a greater risk of a heart attack based on risk factors alone?
| Sir Winston Churchill | Jim Fixx |
| Overweight | Not overweight |
| Not fit | Very fit |
| Heavy smoker | Nonsmoker |
| Died at age 91 | Died of heart attack at age 53 |
From the book Asymptomatic Atherosclerosis
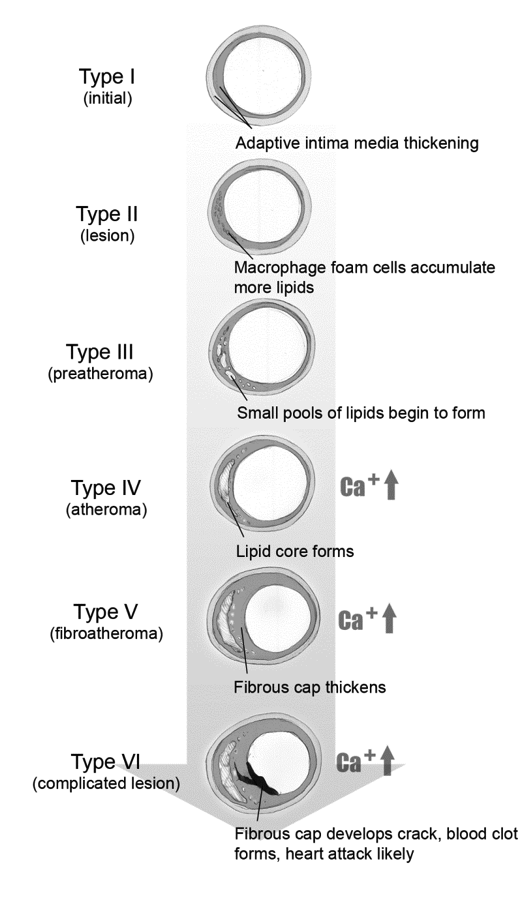
Figure 1 is a simplified schematic of plaque progression. Teenagers have been found to have type 1. Autopsies of young recruits during the Korean War showed fatty streaks, and unfortunately, much younger children today with metabolic syndrome also have demonstrated this. Types IV and higher can rupture, potentially causing a myocardial infarction. The lesions in this illustration do not limit circulation, may not cause symptoms, and could be missed by an angiogram or treadmill test.
Fig. 1 Stages of Plaque Formation
From Dare to Live
Imaging
Imaging can tell us quite a bit about the extent of disease and help us individualize care. The only way to actually see plaque is with an image. A heart scan, angiogram, computed tomography (CT) angiogram, cardiac magnetic resonance imaging (MRI), carotid Doppler ultrasound, carotid intima-media thickness (CIMT) ultrasound, or intravascular ultrasound are all valid imaging technologies. CT angiograms, cardiac MRI and intravascular ultrasound, while valid, are not as widely available, are more invasive, and are less likely to be covered by insurance. So in the interest of time and clinical utility, they will not be discussed here.
Angiogram: This is the most common and most widely available test for the diagnosis coronary artery stenosis.10 Typically, patients get this test if they fail the treadmill test or present for symptoms of acute coronary syndrome. By the time there is an 80% or greater blockage, the patient may be a candidate for an angioplasty, stenting, or bypass surgery. Although an angiogram is good for diagnosing a stenotic artery, it does not detect the earlier stages of plaque. Remember too that most heart attacks are from plaque rupture, not stenosis. Angiograms are appropriate for patients with angina or patients with a high (400 and above) coronary artery calcium score because a narrowed artery can cause blood flow limitation, and subsequent pain and heavy plaque burden increases the chance of the patient having a stenotic artery.11
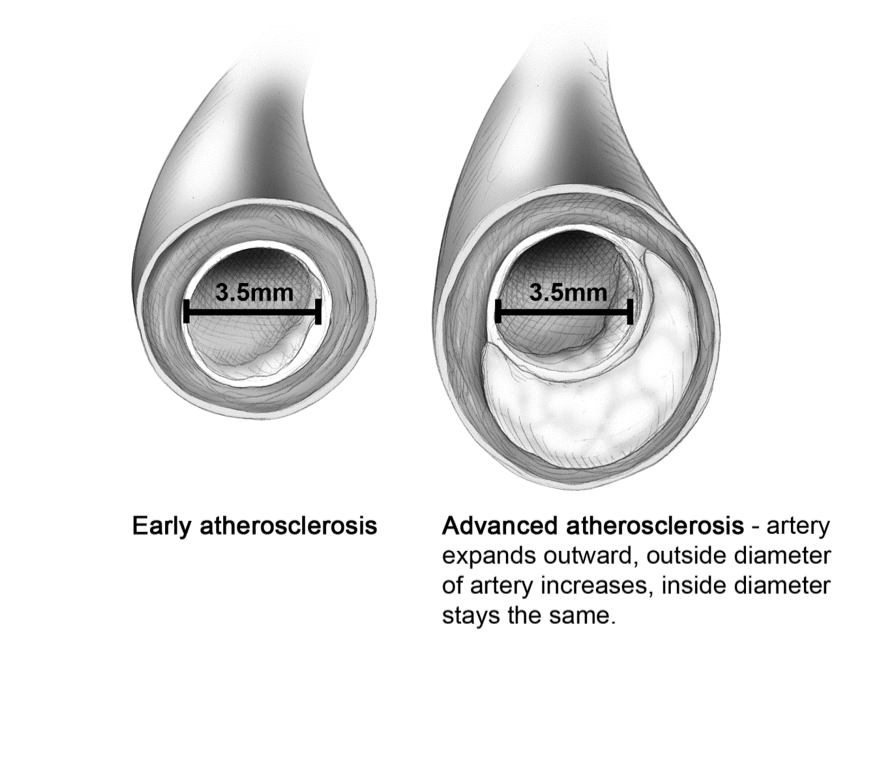
Figure 2 provides an illustration of why a person could have a negative angiogram and a negative stress test but die of a heart attack a week later. The patient’s coronary arteries have expanded outward to accommodate the increase in plaque between the intima and the media. There is no occlusion, so the angiogram should be negative. This artery may contain vulnerable plaque that could rupture, causing a serious myocardial infarction.
Weakness:
- Angiogram will not diagnose those with vulnerable plaque only and no blood flow limitation. The vast majority of all heart attacks are the result of vulnerable plaque ruptures at sites in the artery with minor stenosis and no blood flow limitation.
Strengths:
- Widely available
- Excellent for diagnosis of coronary artery stenosis
Fig. 2 Adaptive Remodeling
Note: An angiogram, echocardiogram [ECG], and stress treadmill can miss this.
Exercise tolerance testing (also known as exercise testing, stress testing, exercise stress testing, or treadmill testing) is used to evaluate patients who have chest pain or chest pain on exertion, sometimes as part of an expanded physical, and patients with known coronary artery disease. Treadmill tests are designed to monitor the electrical activity of the heart during exercise using the standardized Bruce protocol. The patient exercises to 85% of maximal heart rate and is monitored for ECG changes that indicate ischemia or other pathology. ST segment depression (horizontal or downsloping) is the most reliable indicator of exercise-induced ischemia.12
Stress testing has a sensitivity of 78% and a specificity of 70% for detecting CAD.13 It cannot, therefore, be used to rule in or rule out ischemic heart disease unless the probability of CAD is taken into account.14 For example, in a younger low-risk patient, a positive test result is more likely to be a false positive than real, and negative results do not tell us much. In an older high-risk patient, such as those aged over 50 with chest pain, a negative result cannot rule out significant CAD. Exercise testing is therefore of greatest diagnostic value in patients with an intermediate risk of CAD.15
Treadmill tests also don’t do an adequate job of preventive screening for asymptomatic atherosclerosis because although perfusion may be adequate, vulnerable plaque may be present and the patient can still be at risk.16 If myocardial perfusion is adequate and there is no ischemia, preexisting electrical conduction problems, or prior muscle damage (ie, ischemic heart disease) the test usually will be normal.
Adding to the problem is the fact that some patients will form coronary collateral circulation, allowing for adequate myocardial perfusion even though there may be an occluded artery. With collateral circulation, new anastomoses form as an adaptation to myocardial hypoxia. This is common in individuals who have exercised vigorously for many years. This type of patient may have a normal stress test but extensive plaque.
Thus, a high-risk patient with vulnerable plaque may be told he passed his treadmill test but may die of a heart attack the next day from a plaque rupture. Coronary collateral circulation has been recognized for a long time as an alternative source of blood supply to a myocardial area affected by ischemia. More than 200 years ago, Heberden described a patient who had been nearly cured of his angina pectoris by sawing wood each day, a phenomenon called “warm up” or “first effort angina” which was traditionally ascribed to coronary vasodilation with opening of collateral vessels to support the ischemic myocardium.17
Fig. 3 Artery Anatomy
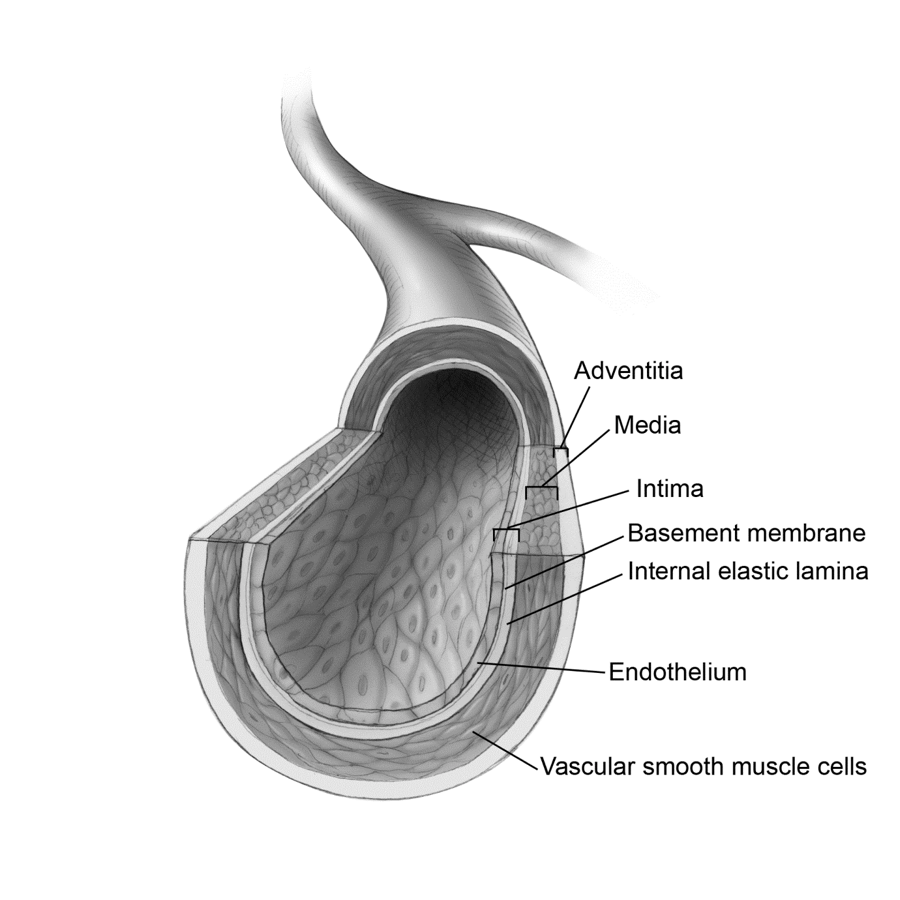
Fig. 4 Area of Interest on CIMT
From Dare to Live
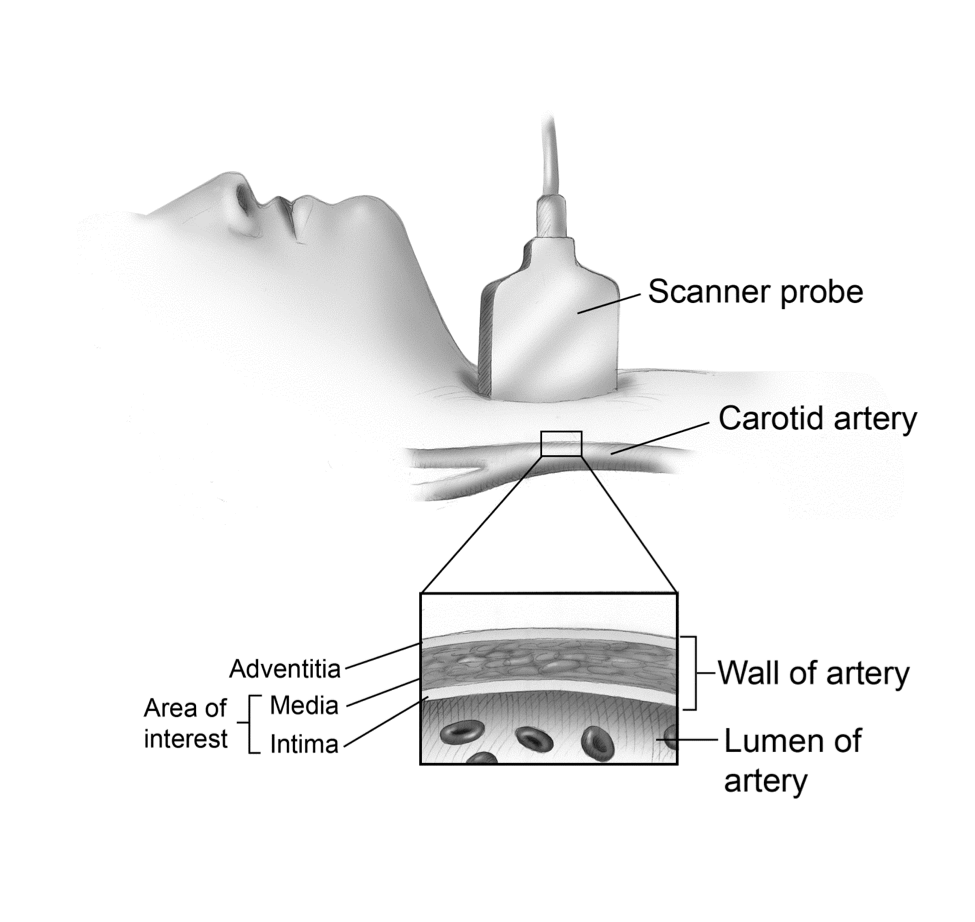
This image shows the sagittal view of the artery.
CIMT: Asymptomatic CAD is common and has been associated with an increased risk for symptomatic CAD. Treadmill testing and angiography do not detect early atherosclerosis well, as discussed previously. Intima-media thickening represents as seen by B-mode ultrasound is the earliest visible change we can see in the arterial wall and is a reliable, safe, and relatively inexpensive means of assessing asymptomatic atherosclerosis.
Increased intimal-medial thickness (IMT) of the common carotid artery as assessed by B-mode ultrasonography is an index of atherosclerosis and is associated with symptomatic CAD.18 In addition, IMT is predictive of cerebrovascular disease,19 left ventricular hypertrophy,20 coronary artery calcium,21 ankle brachial index,22 and albuminuria.23 It is widely accepted that IMT is the only surrogate endpoint for CAD that is endorsed by clinical studies. This is evidenced by the fact that the US Food and Drug Administration recognize IMT as a valid endpoint in the evaluation of new drugs.
Both ultrasound and autopsy studies have found that carotid atherosclerosis correlates well with atherosclerosis elsewhere in the circulation and can be used as a marker of general atherosclerosis.24 Recent guidelines have given CIMT and plaque-based risk prediction a class II A recommendation.3,25
The CIMT determines early atherosclerosis in the carotid artery by measuring the diameter of the media and intima, which is where foam cells accumulate in the early stages of atherosclerosis. In healthy adults, CIMT ranges from 0.25 mm to 1.5 mm. Normal values based on age and gender for black and white races have been established.26 This test is especially useful for asymptomatic patients between ages 40 and 70.
CIMT is different from the standard Carotid Doppler Ultrasound, which measures blood flow velocity. Faster velocity indicates stenosis by virtue of a narrowing of the artery. Unfortunately, in the early stages of plaque formation, there is little narrowing. Thus, a standard carotid Doppler will not identify subtle thickening of the arterial wall and may miss subclinical atherosclerosis and the opportunity to begin treatment early, potentially mitigating the need for a surgical procedure down the road.
Since the 1990s, many statin drug trials have used CIMT for evaluating the regression and progression of atherosclerosis.‡ CIMT has been shown in large-scale, prospective studies (Atherosclerosis Risk in Communities Study,27 Multiethnic Study of Atherosclerosis28) to be an independent predictor of heart attack and stroke. CIMT is more accurate in predicting heart attack and stroke than any other risk factor alone,29 and increased CIMT is an independent risk factor even in the absence of any other risk factors.30
The rate of progression of CIMT is also a good predictor of the risk of actually having a cardiovascular event, such as a stroke or heart attack.31 As little as .03mm of progression per year is significant.
Weaknesses of CIMT:
- Potential Inter-operator variability and full agreement on standardization of the technique. Newer machines are automated and reduce inter-operator variability significantly. These machines can be operated in a family practice or other point-of-care setting and reduce the need for outsourcing.
- May miss coronary artery disease if this is the only test conducted. A low IMT does not preclude the presence of cardiac vessel disease.
- Lack of agreed upon reference set of measurements (normative data) and cut-off points.
- Some lack of standardization on where IMT should be measured.
- Not covered by most insurance companies.
Strengths:
- CIMT ultrasounds may be valuable for patients who are considered to be at intermediate-risk for CHD, as test results can be used to reclassify patients into a higher or lower risk status based on the results of these tests.32
- Intima media thickness of the carotid artery wall represents the cumulative effects of an individual’s exposure over years to all risk factors and provides a snap shot of atherosclerotic disease.33
- It is more sensitive than standard carotid Doppler studies.
- Reclassification of patients as higher risk using CIMT allows earlier treatment, thus potentially reducing the future need for surgery and reducing risk of heart attack and stroke, saving money on the back end.
- Patients reclassified as lower risk need less intervention, saving money on the front end.
- Noninvasive, low cost
- Available in outpatient setting
- Simple
- Accurate
- Related to risk factors
- Predictive of outcomes
- Changes in response to treatment
The Heart Scan
Cardiac CT began as electron beam CT in the early 1980s and continues now with multidetector CT (MDCT). Cardiac CT is a reliable and repeatable means of estimating coronary artery plaque burden.
There are 2 types of cardiac CT available, electron beam tomography (EBT) and MDCT (also known as UltraFast CT, 64 slice, or Multi-slice CT). EBT uses a low-radiation, high-speed electron beam to scan the heart noninvasively for the presence of calcium deposits. EBT is capable of very rapid imaging of cardiac anatomy. Between 1985 and the late 1990s, hundreds of papers were published from around the world validating the cardiac application of EBT. MDCT is promoted as an alternative to EBT. However, MDCT imaging involves considerably more radiation exposure, and this should be taken into consideration when choosing which coronary artery calcification (CAC) procedure to recommend. Both types of heart scans identify calcified plaque and give a direct indication of coronary plaque burden. CAC also can be used to track progression or regression of plaque. This is particularly useful for clinicians when evaluating efficacy of treatment.
EBT uses a rotating electron beam to acquire 64 slice, 50-100 ms x-ray images at 3 mm intervals during 30-40 second breath-hold. Any cardiac scan using less than 64 slice technology is not accurate enough.34
CAC is almost always indicative of atherosclerotic plaque, and a strong relationship has been established both through histological and intravascular ultrasound studies. CAC provides a useful estimate of total coronary plaque burden.35 CAC is known to occur as a part of the atherosclerotic process and is an active process resembling bone formation within the vessel wall. The process of CAC involves osteoblast-like cells, cytokines, transcription factors, and bone morphogenic proteins.36
Serial EBT scanning has demonstrated that lipid-lowering therapy can slow progression of atherosclerosis.37 In the St. Francis heart study, individuals with an initial CAC score in the 100–400 range had a 10-fold increase in relative risk compared to those with a zero score. A CAC score of 100 is generally thought to be the dividing line between mild and moderate atherosclerosis.38 Recently, calcium scoring was shown to improve risk stratification, especially in patients in the intermediate-risk category.39
Approximately 25% of individuals will be reclassified (higher or lower) after a heart scan.40 The CAC score detected by CT is a stronger predictor of cardiovascular events and mortality than conventional risk factors alone.41 The rate of growth per year is indicative of the extent of the disease process. Newer plaque has less calcium and is more prone to rupture than older calcified plaque, so both calcium burden and extent of disease progression should be taken into account. A progression rate of 15% or less per year is considered by experts to indicate stabilization of disease and is associated with a good prognosis.42
Calcium score is typically given as either an Agaston or calcium volume score. Either can be used for serial measurements of plaque progression; however, calcium volume score is thought to be more reproducible for tracking progression or regression of atherosclerosis.43
The question of what to do with patients with zero scores arises frequently. Zero score simply means they have not yet developed detectable calcified coronary plaque but they may still have fatty streaking or the early stages of plaque. The event rate in patients with zero scores is very low (approximately 0.11%–12%).44
Increasing coronary calcium scores indicate higher risk for CAD in both asymptomatic and symptomatic patients.45 The CAC score is directly proportional to the total plaque area, although calcium makes up only about 15% to 20% of the total plaque burden. Exceptions include patients that have non-calcified plaque, such as young smokers.
The CAC content is indicative of plaque burden but not of the degree of stenosis. Typically, the score will be given as a percentile based on age and gender. The most widely used method for CAC quantification is the Agatston score.46 The report also will indicate where the plaque is.
Atherosclerosis is a slowly progressing systemic multifocal arterial disease with focal manifestations caused by stenotic and or thrombosis prone vulnerable plaques. Multiple areas of plaque indicate diffuse disease. One or 2 areas of moderate to high CAC indicate a higher plaque burden in a given artery segment. Some investigators have reported higher event rates in patients with more diffuse disease.
It is suggested that a follow-up scan be conducted in 12 months for those in the 40th percentile§ or above, in 3 years for those in the 25th to 40th percentile, and in 10 years for those in the 15th or lower percentile if below age 40. If patients are status post-angioplasty, the scan also can be used to monitor progression or regression and treatment effectiveness.
Summary
Not all CAD is symptomatic. Asymptomatic atherosclerosis typically progresses over time. Atherosclerosis (symptomatic or not) may lead to plaque rupture and subsequent MI or stroke. Heart scans and CIMT detect early subclinical atherosclerosis.47,48 The limitations of current cardiovascular risk stratification guidelines are recognized by the American Heart Association, The National Cholesterol Education Program Expert Panel, and The European Third Joint Task Force.49 The use of noninvasive screening tests to identify asymptomatic atherosclerosis provides an option for advanced risk assessment, particularly in those who were judged to be at intermediate risk.50 Primary care practitioners are well positioned in the healthcare system to engage in preventive cardiology.
The CIMT is best for detection of early asymptomatic atherosclerosis and especially stroke risk. Most hospitals now have 64 slice ultrafast heart scans available. Stand-alone cardiac CT facilities also exist. Carotid intima thickness is becoming more available in private practices. Insurance reimbursement is becoming more common. Both of these tests are better than any serum markers or epidemiological algorithms at determining risk. A heart scan and a CMIT may be done together because some people have low levels of plaque in their carotid arteries but a high plaque score in the coronary arteries and vice versa. It’s better to know than to guess.
*According to the CDC, heart disease is the number-one cause of death for those in the 65 years old and over group, number 2 for those between ages 45 and 65, and number 3 for those between ages 35 and 45.
†Some tests, though helpful, are expensive, overly invasive, or difficult to obtain (eg, computed tomography angiogram, intravascular ultrasound imaging technologies).
‡FDA approval of some of these drugs has hinged on demonstration of plaque reversal based on CIMT.
§ As an example, 40th percentile means that 60% of age-matched controls have more coronary calcium and 39% have less. The imaging lab typically will give you this data.






