Abstract
Sleep is an essential part of our everyday lives and can have a significant impact on health.1 It takes up one-third of the day and needs to be understood and respected for the importance it brings to our patients. There are many sleep disorders that have been recognized and are too numerous to review in this article.2 A detailed explanation of all sleep disorders can be found in the classification of sleep disorders, well-outlined by the American Academy of Sleep Medicine.3
In the first section of this article, sleep patterns and stages will be reviewed and summarized with any health information noted. To better understand the impact of sleep on health, one needs to understand basic sleep architecture. The first section will outline in detail the structure of sleep staging and significant correlations to health.
The next section will focus on the patient assessment of sleep quality and recognizing potential sleep disorders in primary care visits. The focus is on evaluating sleep disorders as a primary care practitioner. It is estimated that 50 million to 70 million people in this country alone have a chronic sleep disorder.4 This makes assessment of a patient’s sleep an extremely essential part of the primary care responsibility. Treatment will be discussed very minimally in this article.
Normal Sleep Cycle
The 2 larger sleep stages are rapid eye movement (REM) sleep and nonrapid eye movement (NREM) sleep. NREM sleep is further classified as stage 1, stage 2, and stage 3 (stage 4 is no longer used). Stages 3 and 4 sleep used to be referred to collectively as delta sleep or slow-wave sleep (SWS) and were classified as stages 3 or 4 sleep depending on the percentage of delta waves in a 30-second time interval.5
From wakefulness, an individual will begin with light sleep, which is classified as stage 1 sleep. An example of light sleep is while driving a vehicle, one experiences “drowsiness.” This is when brain waves typically found in stage 1 sleep are prevalent.6 Normal sleep involves repeating sleep stage cycles 4 to 5 times during the night (Figure 1).
REM sleep is known as dream sleep and is characterized by low-amplitude, mixed-frequency brain waves, rapid eye movements, and decreased muscle tone, also referred to as muscle atonia.7 The average adult spends around 20% to 25% of the total sleep time in this stage of sleep, which decreases slightly with age.8 There are usually 4 to 5 cycles of REM sleep, with each REM period lasting for longer periods as the night progresses (Figure 1).9 Due to the muscle atonia during REM sleep, obstructive sleep apnea (OSA) has been found to be more frequent during this stage of sleep.10 OSA is usually more prominent while people sleep supine, so theoretically OSA might be more serious during REM sleep when the patient is sleeping supine.11 This is clinically significant because if the patient is being tested for sleep apnea and body position and sleep stages are not recorded, the results may not be representative of the severity of the OSA.
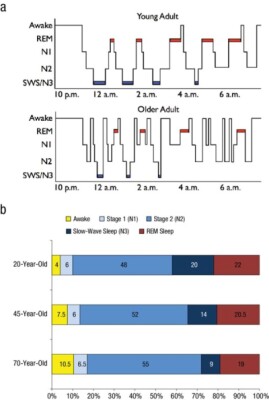
Figure 1. Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: integrating a half-century of multidisciplinary research. Perspect Psychol Sci. 2015;10(1):97-137. Used with permission from author.
REM Behavioral Disorder (RBD)
REM-related behavior disorder, which was first reported in 1986,12 is relatively uncommon in the general population (around 0.5%), but its prevalence increases up to 13% in individuals over the age of 60.13 RBD individuals act out their dreams, whereas those without the disorder have an active visual of the dream but stay in the paralyzed state during sleep. Our brain controls postural atonia, and during REM sleep, there is a loss of postural muscle tone, so humans generally will dream visually of events or activities, but their body will not act out the dreams. In RBD, an imbalance in the brainstem results in the loss of REM muscle atonia.14 In other words, the REM-sleep muscle activity in people with RBD is “tonic,” or activated instead of being inhibited.
Individuals with RBD have an increased risk of neurodegenerative diseases including dementia. In a study of 174 patients diagnosed with RBD between 1991 and 2013, a significant number of those patients (37.4%) were subsequently diagnosed with either Parkinson disease (PD), dementia with Lewy bodies, multiple-system atrophy, or mild cognitive impairment.15 RBD is common in patients with PD.16 Research at the University of Texas Southwestern Medical Center revealed that RBD was correlated with increased progression of PD (P<0.001) and could potentially be used to begin therapy.17
Dreams can be aggressive or violent in nature, an inherent concern for those who suffer from RBD, though some consider this type of behavior more of an exception than a common feature of RBD.18 Regardless, RBD is concerning and, if associated with aggressive behavior, requires intervention. This article will review the use of the drug clonazepam and the hormone melatonin for the treatment of RBD. There are no known behavior interventions that might help with this disorder; however, it is important to maintain a safe environment for those suffering from RBD. Protective padding on the floor and sleeping in a separate bedroom from their partner are all considerations to add safety.19
Clonazepam and RBD
Clonazepam, a benzodiazepine, and melatonin have traditionally been considered first-line treatment interventions for RBD. However, clinical trials have failed to support this approach.20 Clonazepam is a long-acting benzodiazepine that acts as a gamma-aminobutyric acid A (GABAA) receptor agonist. The drug also enhances the synthesis of serotonin.21 The problem with clonazepam is the side-effect profile for older adults as listed in the Beers Criteria.22 Increased sedation, fatigue drowsiness, motor impairment, and risk of falling are some of the most common side effects of this medication. In addition, the use of clonazepam is contraindicated in those with liver disease.21 In a meta-analysis, the use of benzodiazepines increases the risk of fractures (P<0.001).23 There is no evidence that clonazepam reduces the risk of developing neurodegenerative diseases or dementia.
Melatonin, RBD, and Parkinson Disease
Drugs that affect the sleep-wake cycle or circadian rhythm in humans can be classified into 3 areas. First, there are the sedative hypnotics used to treat insomnia and help initiate and maintain sleep. Benzodiazepines, such as clonazepam and lorazepam, and nonbenzodiazepines, such as zolpidem, are in this category. The second class of drugs, the stimulant medications, enhance wakefulness or alertness. Drugs in this class include armodafinil, atomoxetine, and methylphenidate. The third group, the chronobiotic drugs or substances, are meant to realign or reset the circadian rhythm.24 Melatonin is a hormone that acts as a chronobiotic agent, and this is traditionally used in the treatment of insomnia.
Melatonin has been used for years, and dosing for insomnia is usually 1to 6 mg at bedtime.25 Melatonin can be used in combination with clonazepam or as a second-line monotherapy in the treatment of RBD. In a small RBD study, the use of 3 to 9 mg of melatonin nightly significantly reduced the number of “tonic” REM activity events (P<0.01). Thirteen of the 14 subjects reported subjective improvement in RBD symptoms.26 One hypothesis of why melatonin works in RBD is that it resynchronizes the biological and circadian rhythms.27
Patients with Parkinsons disease have disturbances in biological rhythms such as heart rate, temperature, and hormonal fluctuations. It has been shown that patients with PD have elevated levels of cortisol at night and in the early morning.28 Individuals with PD develop rigidity, postural instability, and gait disturbances that can be difficult to manage. These patients also exhibit mood and sleep disorders that can lead to a decline in the quality of life. Patients with PD have an almost 6-fold increased risk of developing dementia, especially as they age.29 The cause of PD is complex and not fully delineated. PD has been linked to environmental toxin exposure, loss of dopaminergic neurons, inflammation, and oxidative damage.30
Sleep disruption and difficulty initiating and maintaining sleep are major problems in patients with PD. In a metanalysis of 7 studies looking at the benefits of melatonin for sleep disturbances in those with PD, melatonin made significant improvements in the objective and subjective quality of sleep (P=0.001).31
Melatonin possesses cytoprotective qualities and has been shown to reduce oxidative stress.32 Melatonin is synthesized primarily in the gastrointestinal tract from tryptophan, though it is also produced inside cells throughout the body and is able to cross the blood-brain barrier (BBB).33
Oral bioavailability rates vary but are relatively low, in the range of 3% to 33%.34 Doses in human trials have used as much as 100 mg daily without any signs of toxicity.35 One author suggested that if animal studies using melatonin were translated into human doses, the dose of melatonin needed to be cytoprotective would be in the range of 40 to 100 mg per day.36 The author recommended that human clinical trials are needed using these higher doses.
NREM Sleep
Stage 1 NREM Sleep
Stage 1 sleep is characterized by mixed alpha and theta brain waves and rolling eye movements. This stage of sleep is a transition between wakefulness and sleep. Stage 1 sleep, as well as wakefulness after sleep onset, increases with age.37
Stage 2 NREM Sleep
Stage 2 sleep has characteristic theta waves, along with sleep spindles and K-complexes on the electroencephalogram (EEG). (Figure 2.) Sleep spindles help maintain sleep and reduce disruptions from external stimuli by blocking sensory information to the cerebral cortex.38 Although not completely understood, sleep spindles may help with memory and learning consolidation and are associated with increased neuroplasticity.39 Neuroplasticity is the ability of the nervous system to change its activity in response to intrinsic or extrinsic stimuli by reorganizing its structure, functions, or connections.40 Sleep spindles are increased in individuals participating in learning exercises and can be further distinguished as slow and fast.41 The slow spindles are at a frequency of 10 to 13 Hz and fast at 13 to 16 Hz.
Sleep spindle activity is disrupted in Alzheimer disease (AD), with fewer spindles during NREM sleep and reduced fast-spindle activity. This finding on sleep recordings (polysomnogram) might serve as an inexpensive diagnostic tool for AD.42 Research has shown reduced spindle activity in the frontal brain in children with autism spectrum disorder (ASD). Research has shown that children with ASD have a dysfunction in connectivity between different areas of the brain, and this may be associated with reduced spindle activity.43 One could theorize that altering the spindle density or type through specific learning exercises may provide functional improvements in individuals with AD or ASD.
An increase in fast-spindle frequencies appears to be related to learning new motor skills. In a small study of 22 students given motor-skill exercises prior to sleep, the number of fast spindles increased significantly, and this was linked to skill improvement in the students (P<0.01). The number of sleep spindles decrease with normal aging.44
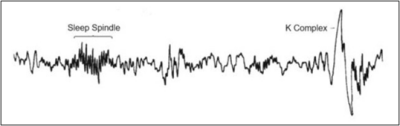
Figure 2. Farooq H, Jain A, Sharma VK. Stage 2 sleep is characterized by the appearance of both sleep spindles and K-complexes. Int J Res Eng. Sci Manag. 2021;4(1):56-59.
In a study using 32 college students, 3 groups were exposed to 3 different oscillating white noise (OWN) frequencies (12, 15, and 50 Hz, respectively) during stage 2 sleep and throughout daytime naps. The OWN at 15 Hz was associated with the largest increase in fastspindle activity compared to the other 2 frequencies (P<0.001).45
In a study looking at individuals with PD suffering from dementia, sleep spindles were decreased compared to nonPD individuals. The authors suggested this finding might serve as a diagnostic marker for those individuals with PD who could later develop dementia.46
Parkinson disease was mentioned earlier as being associated with increased prevalence of RBD. Patients with PD may also have sleep-spindle changes associated with increased amounts of exercise. There is evidence that intervention with movement exercise in patients with PD improves memory and is associated with an increase in sleep-spindle density (P=0.0413).47
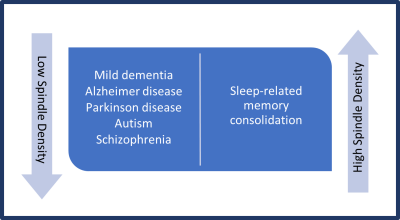
Figure 3. Low sleep spindle density is associated with mild dementia in Alzheimer disease, Parkinson disease, autism, and schizophrenia.48
Interventions for Sleep-Spindle Density Enhancement
Zolpidem
GABAA modulators such as zolpidem and eszopiclone have been shown to increase sleep-spindle density, and researchers have studied their effects on memory.49 These medications are used for insomnia and carry a warning for overuse and dependance, though they are nonbenzodiazepines.50 In a small trial of 22 individuals, better memory performance was found in those taking zolpidem vs placebo (P=0.034).51
Agomelatine
Agomelatine is a melatonin receptor agonist, approved in Europe for the treatment of depression. It is a melatonin receptor 1 and 2 agonist and releases norepinephrine and dopamine via serotonin receptor antagonism.53 It is not available in the United States. Agomelatine has been shown to increase sleep-spindle density in clinical trials using polysomnographic recordings.53
Valeriana officinalis
In an animal study using 400 mg/kg of valerian on neuropathy in rats, there was an increase in sleep-spindle density after 3 and 6 days (P<0.05).54 Valerian is reported to be like a benzodiazepine medication because the herb binds to the GABAA receptors; however, valerian binds to a different subunit on the receptor.55 No human trials can be found by this author.
Stage 3 NREM Sleep
Slow-wave sleep used to be comprised of stages 3 and 4 depending on the amount of slow-wave activity (SWA) or delta waves present during specific 30-second intervals.56 Current terminology has grouped both SWS stages into 1, thus standardizing sleep stages to 4 in total.57 SWS or SWA is associated with physiologic changes such as increased growth hormone production and increased parasympathetic activity.58 SWS decreases with age and is less in women than in men.59 Vigorous exercise can reduce the amount of time it takes to fall asleep and increase the amount of SWS.60
Night Terrors and Sleepwalking
Sleepwalking and night terrors occur during SWS.61 According to research, the cerebral cortex allows physical movement (eg, walking) and open eyes during SWS, but the part of the brain that controls judgement and memory are inactive.62 When a person experiences a night terror, no recall or memory occurs from the event, unlike a nightmare, in which there is usually recall of a dream. The etiology of night terrors is unknown.63 There is no specific treatment for night terrors and sleepwalking; however, this situation usually does not occur past 10 years of age. Reassurance and safety measures for sleepwalking are the mainstays of treatment.
Sleep Fragmentation and Wake After Sleep Onset
As we age, older individuals spend more time in bed awake compared to younger adults and children. By the time one reaches the age of 65, the total amount of time in slow-wave sleep is lower, and the amount of time of wakefulness after sleep onset (WASO) is higher (Figure 4).
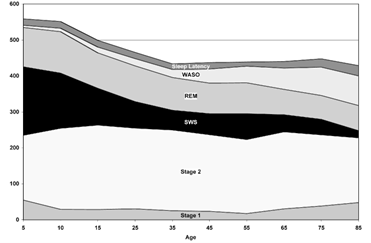
Figure 4: Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255-1273. Acronyms: Wakefulness after sleep onset (WASO); slow-wave sleep (SWS); rapid eye movement (REM).
When a patient over the age of 65 years presents with disrupted sleep, the most important medical approach is to rule out a common sleep disorder such as obstructive sleep apnea (OSA), restless leg syndrome (RLS), and periodic limb movements of sleep (PLMS). Chronic medical conditions may affect initiation and/or maintenance of sleep and must be considered when treating the patient.64 Sleep fragmentation and decreased sleep quality have also been reported because of overexposure to blue light emitted by electronic devices.65
There is an increased risk of developing AD for those who have a higher level of sleep fragmentation. In a study of 700 older individuals without dementia, an objective measure of increased sleep fragmentation was associated with a higher risk for the development of AD (P=0.02).66
Sleep Time and Health
According to the latest poll (2023) of the American population, the average total sleep time in the US is less than 6 hours.67 Shortened sleep time can be linked to an increase in negative health outcomes and an increase in overall mortality rates.68 In a meta-analysis by Grandner et al, people with reported sleep durations of between 7 to 8 hours had the lowest overall mortality risk compared to those who slept less than 6 hours and greater than 9 hours.69,70
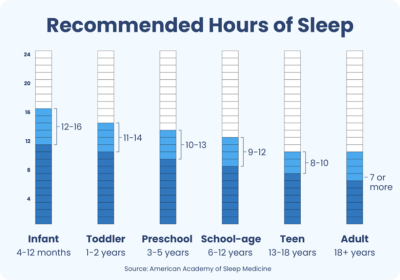
Hirshkowitz M, Whiton K, Albert SM. National Sleep Foundation’s updated sleep duration recommendations: Final Report. Sleep Health. 2015;(1)4:233-243.
Sleep Assessment in Primary Care Setting
In general, primary care physicians fail to adequately assess sleep quality and sleep disorders in practice.71 Healthcare practitioners need to identify potential sleep disorders during the initial consultation with patients, as well as explore the causes of symptoms of insomnia, daytime sleepiness, and fatigue. In addition, the practitioner must inquire about the possibility of RBD. A standard series of questions regarding sleep and sleep hygiene should be a customary practice in any review of symptoms.
Insomnia
The Diagnostic and Statistical Manual of Mental Disorders (DMS-5) defines insomnia as difficulty initiating sleep, difficulty maintaining sleep, or experiencing early morning awakenings.72 This definition in clinical practice excludes those situations related to coexisting medical conditions, psychiatric illness, or physiological conditions such as drug or alcohol abuse.
A basic approach to insomnia is to first determine whether the problem is difficulty initiating sleep, maintaining sleep, or both. Acuteness or chronicity is an important factor when evaluating the cause of the patient’s insomnia. The etiology of chronic insomnia can be complex, and one needs to consider multiple causes. In a study of 358 men and women with complaints of insomnia, the largest percentage of those participants reported that work- or school-related stress, depression, pain, and medical illness accounted for their sleep problem (P<0.0001).73
There can also be some differences based on gender that require consideration. For example, having trouble falling asleep following a traumatic event such as a death, physical or mental trauma, or a major change in sleep-wake habits is not unusual; however, sleep disturbances are more common in females compared to males. In a small study of 35 individuals 1 month after a traumatic event, women were more affected by sleep problems than men, and women were found to have a more significant reduction in total sleep efficiency, increased amount of wakefulness after sleep onset, and reduced total sleep time (P<0.05).74
Evaluation of Sleep Apnea
It is estimated that up to 17% of people in the United States suffer from obstructive sleep apnea.75 OSA can be defined as a partial or complete obstruction in the airways lasting at least 10 seconds in duration. The diagnosis of OSA is made when at least 5 of these events occur during each hour of sleep, as measured on the the apnea/hypopnea index (AHI). Symptoms of obstructive apnea include snoring, making gasping sounds during sleep (witnessed apnea), frequent awakenings, headaches, and daytime sleepiness. OSA is often accompanied by drops in saturation oxygen (SaO2) during sleep. A good tool to screen these patients is the STOP-BANG questionnaire. The questions are answered yes (1 point) or no (0 points). Having a bed partner present during the visit is very helpful when completing any sleep questionnaire. A score of 0 to 2 would suggest a low risk of having OSA. A maximum score of 10 would suggest a very high risk of OSA.76
S = “Do you snore loudly?”
T = “Do you feel tired or sleepy during the day?”
O = “Has anyone observed apneas or choking during sleep?”
P= “Do you have high blood pressure?”
B = Does the patient have a body mass index (BMI) > 35?
A = Is the patient older than Age 50?
N = Is the patient’s Neck circumference ≥17 inches (men); ≥16 inches (women)?
G = Is the patient of the male Gender?
OSA is more common in overweight, middle-aged men, though it is prevalent in other populations as well. Because OSA is associated with airway obstruction, there is a positive correlation between neck circumference and neck-to-height ratio and the risk of OSA.77 The primary care practitioner can start with something as simple as an overnight oximetry test to look for patterns of oxygen saturation consistent with OSA. To best evaluate OSA, an overnight in-house polysomnography (PSG) provides a detailed analysis of sleep stages, body position, heart rate, airway, diaphragm breathing patterns, and awakenings throughout sleep. During this testing, if the patient has significant OSA during the first half of the night, nasal continuous positive airway pressure (CPAP) can be initiated. This saves the patient from making 2 appointments and is referred to as a “split-night sleep study” and is used only when the AHI is significant.
Evaluation of Restless Leg Syndrome and Periodic Limb Movements of Sleep
Restless leg syndrome is an overwhelming, uncontrolled urge to move the legs. RLS can also present with unusual sensations to the legs and is often referred to as a “creepy crawling” sensation by patients. The condition is most problematic and symptomatic in the evening and can be associated with a condition called Periodic Leg Movements of Sleep. PLMS is when the limbs make jerking movements every 10 to 40 seconds throughout sleep. This condition can be more problematic if it causes arousals during sleep, which may result in more daytime sleepiness. Many patients suffering from RLS also have PLMS; however, only around 30% of patients with PLMS have symptoms of RLS.78 RLS is a clinical presentation, and PLMS requires an overnight polysomnogram to diagnose.
Evaluation of Daytime Sleepiness and Fatigue
It is important in practice to differentiate between daytime sleepiness and fatigue. Feeling tired or fatigued during the day is not the same as falling asleep during the day. Falling asleep throughout the day is medically referred to as excessive daytime somnolence (EDS) or hypersomnolence and can be evaluated initially using a questionnaire called the Epworth Sleepiness Scale (ESS).79 Used for years by sleep centers, it can help make the distinction between fatigue and pathological sleepiness. Any patient in the primary care setting with a complaint of daytime sleepiness would benefit from having the primary care practitioner administer this questionnaire.
As we age, older individuals spend more time in bed awake compared to younger adults and children.
With the ESS questionnaire, the patients rate on a scale of 0 to 3 how likely they are to fall asleep in different situations. A rating of 0 means they would never fall asleep, and a 3 reflects a high likelihood of falling asleep. The maximum score could be as high as 24, which represents a serious problem. It must be stressed that this tool is helpful to assess the degree of EDS but is not diagnostic.80
The 8 individual questions include falling asleep while:
- Reading while sitting down
- Watching TV
- Inactively sitting in a public setting
- Sitting in the passenger seat of a car for one consecutive hour
- Lying down to rest in the afternoon
- Sitting and talking with another person
- Sitting quietly after eating lunch without having consumed alcohol
- Sitting in the driver’s seat of a car while stopped for a few minutes in traffic
One condition that warrants a closer look when there is significant daytime sleepiness is narcolepsy. Patients with narcolepsy will present with symptoms of significant daytime sleepiness and even report falling asleep while talking to others. Narcolepsy is broken down into 2 types: type 1, which involves having cataplexy, and type 2, which is not associated with cataplexy. Cataplexy is the loss of muscle tone or muscle weakness brought on by strong emotions such as anger, laughter, or excitement. Although 70% of patients with narcolepsy have cataplexy, narcolepsy without cataplexy is less understood.81 Type 1 narcolepsy is thought to be an autoimmune condition caused by a deficiency of orexin-producing neurons in the lateral hypothalamus.82 The typical age of onset is in the late teens and early adulthood. Although a high score on the ESS questionnaire can help in assessing the probability of narcolepsy, it cannot be used to diagnose this disorder, especially type 2 narcolepsy.83
There is a low prevalence of this disorder, which makes it difficult to assess and diagnose. It requires obtaining a careful history with objective testing using overnight polysomnography followed the next day by multiple sleep latency testing (MSLT). The MSLT is a series of 5 naps during the day every 2 hours following the overnight PSG. The patient is given 20 minutes to fall asleep at each nap and if they fall asleep within that period are then allowed to sleep for 15 minutes.84 Diagnostically, patients with narcolepsy will fall asleep an average within 5 minutes during nap sessions and go into REM sleep 2 of the 5 naps. A normal individual will either not fall asleep during naps or take longer than 10 minutes to fall asleep. Without the use of this objective testing, a risk of misdiagnosis is possible. In a study evaluating 41 patients diagnosed with narcolepsy, only 46% of those were found to have narcolepsy. Of the study group, 44% of the group were diagnosed by family practitioners. One of the main reasons for misdiagnosis was the lack of objective testing using PSG and MSLT.85
Summary
The prevalence of patients coming into the office with sleep problems in general family practice is significant. Having a basic understanding of sleep architecture and the significance of sleep health in our patients can help improve the quality of patient care. General practitioners need to take the time to better understand these types of problems and investigate the impact on their patients. Making an accurate diagnosis or identifying the cause of sleep problems is a good first step in providing patients with accurate and meaningful treatment plans. Appropriate referral to a sleep clinic or specialist is essential for a more complete diagnosis and, thus, treatment.






