Abstract
Arum palaestinum Boiss. is a time-honored botanical in Traditional Arabic Palestinian herbal medicine, where it has been used to strengthen bones and treat cancer, parasites, infections, and many other maladies. Recent work demonstrates anticarcinogenic action both in vitro and in vivo, and that work is coupled with a proof-of-principle mechanism of action data showing induction of the pro-apoptotic protein, caspase-6. The data to date is strongest for an Arum palaestinum extract that has been fortified with isovanillin, linolenic acid, and β-sitosterol, constituents that are endemic to a crude water extract of Arum palaestinum. Safety data regarding toxicity are encouraging. Acute dosing animal studies and in vitro studies, which compare effects on cancerous and healthy cell lines, show toxicity thus far limited to cancer cells. Future phase 1 and phase 2 clinical trials are necessary to fully understand pharmacokinetics in humans and to potentially demonstrate clinical efficacy in human populations.
Introduction
Physical characteristics and taxonomy
Arum palaestinum Boiss. is a flowering perennial species within the family Araceae, also known by its common name as Solomon’s lily,1 and often referred to in literature as black calla lily.2 Arum palaestinum’s membership in the larger botanical family of Araceae is significant from an ethnobotanical perspective, as this family is coming to be viewed as a particularly rich source of medicinal botanicals.3
Arum palaestinum is included in the genus Arum L, along with Arum italicam Mill. (commonly known as Italian lords and ladies), and Arum maculatum L (known as cuckoo pint).4 Species of Arum have been in the Mediterranean region for millennia, and are represented in engraved drawings in the temple of Thutmose III in Karnak as plants that were brought to Egypt from Canaan in 1447 BCE.5
Arum palaestinum is recognizable by its red-brown/purple spadix and spathe of dark purple (Figure 1). The arrangement of its leaf blades speaks to a commonly used, aptly descriptive name in Arabic that translates to “elephant ear” (Figure 2),5 while its seeds are identifiable by their vibrant red color (Figure 3).

Figure 1. The purple spathe and reddish spadix of Arum palaestinum.

Figure 2. Characteristically shaped leaves of Arum palaestinum.5

Figure 3. Brilliant red seeds of Arum palaestinum.
Arum palaestinum as a food-medicine
Arum palaestinum has an eclectic history as both a food and a medicine. As is often the case, its use does not fit neatly into one or either category exclusively, but rather reflects its wide use as a food-medicine. According to Yaniv,5 the de-stemmed leaves, cooked with lemon or sorrel, are considered a delicacy by Arabs, who also traditionally esteem the plant as a medicine for the treatment of cancer, for the killing of worms in animals and humans, as a means to strengthen bones, as a treatment for infections in open wounds, and as a treatment for kidney stones. Additional sources confirm its use as a traditional Arabic medicine in the treatment of cancer, internal bacterial infections, poisoning, and disorders of the circulatory system, and refer to Arum palaestinum as a botanical used in Traditional Arabic Palestinian herbal medicine.6,7 Among Jews in Iraq, Arum palaestinum is revered as a treatment for skin sores, syphilis, rheumatism, tuberculosis, diarrhea, and stomach worms.5
The measurable anticarcinogenic effects of the fortified extract of Arum palaestinum are accompanied by an understood mechanism of action that could apply across multiple types of solid tumors.
According to a 2008 ethnobotanical study of edible plants within 5 rural districts of the Palestinian Authority, where preservation of the traditional knowledge of wild edible plants would be expected to be best maintained, Arum palaestinum was identified as one of the species rated highest for its cultural importance, a reflection of the diversity of ways in which an item is used as a food (eg, a vegetable, an herbal tea), and was cited by over half of those surveyed as a wild plant used for a food purpose.8 Consistent with a combined food-medicine use, Arum palaestinum is described in this survey as a food that is prepared by the leaves boiled in water, fried in olive oil, garnished with lemon, and consumed because of the belief that the plant helps prevent colon cancer. Also, in terms of contemporary use as a Complementary/Alternative Medicine (CAM), a 2011 questionnaire administered to a Palestinian cohort of 372 patients with cancer found that 43.5% of the cohort reported use of Arum palaestinum, making the plant the most commonly used CAM therapy among the cohort.9
Materials and Methods
To identify phytochemicals in Arum palaestinum reported to exert anticarcinogenic action, the author conducted a review of the peer-reviewed literature, using PubMed Central (PMC) and PubMed and the following search terms: Arum palaestinum, black calla lily, cancer, ethnobotany, and Traditional Arabic Palestinian herbal medicine. The author also reviewed the recently published in vitro and in vivo literature related to the anticarcinogenic activity of an extract of Arum palaestinum fortified with isovanillin, linolenic acid, and β-sitosterol, constituents that are endemic to a crude water extract of Arum palaestinum. Finally, the author reviewed mechanism of action and safety data published to date.
Results
Chemical constituents
Through rather extensive analysis of Arum palaestinum via liquid chromatography tandem-mass spectrometry (LC-MS), combined with previously published literature, Abu-Reidah10 reports the presence of 180 phytochemicals (with tentative identification), including 53 flavonoids, 33 phenolic acids, 10 terpenoids, 7 iridoids, and 6 amino acids, obtained from a hydro-methanolic extract. With respect to the various chemical categories obtained through aqueous vs methanolic extraction, Jaradat11 informs us that the aqueous extract is characterized by the presence of saponin glycosides, carbohydrates, phenols, tannins, and flavonoids, while methanol extraction retrieves alkaloids, in phenols, and flavonoids (though specific phytochemicals are not given for each category). In comparison, analysis of the ethyl acetate fraction by El-Desouky revealed the presence of the polyhydroxy alkaloid compound (S)-3,4,5-trihydroxy-1H-pyrrol-2(5H)-one, caffeic acid, isoorientin, luteolin, vicenin 11, and the rare compound 3,6,8-trimethoxy, 5,7,3',4'-tetrahydroxy flavone.12 Further work by Afifi13 demonstrates the presence of 2 flavone C-glucosides, specifically isoorientin (luteolin 6-C-glucoside) and also vitexin (apigenin 8-C glucoside). Finally, the recent work by Cole highlights the presence of isovanillin, linolenic acid, and β-sitosterol in Arum palaestinum, with enhanced levels of these constituents creating a fortified water extract with enhanced anticarcinogenic potential, as will be discussed in further detail.14 A nonexhaustive summary of many compounds of interest that have thus far been attributed to Arum palaestinum, by main category and reference, is presented in Table 1.
Table 1. Notable Constituents of Arum palaestinum
| Reference (Chemical Category) | Constituenta |
Abu-Reidah10 (Hydro-methanolic extract) | |
Flavonoid/flavonoid derivatives | esculetin-O-hexoside, quercetin dihexoside, 6,8-Di-C-β-glucosylapigenin (Vicenin 2),15 di-C-glucosylluteolin, lucenin-2, vitexin-O-glucoside, quercetin-O-rhamnosylrutinoside, kempferol-O-rutinoside-O-glucoside isomers, isoshaftoside, shaftoside, Di-C-glucosylluteolin, kaempferol trihexoside, cyanidin-3-rutinoside,16 diosmetin-6,8-di-C-hexose, (epi)catechin,17 isoorientin,18 orientin,19 pseudobaptigenin-O-hexoside (rothindin), aromadendrin-O-hexoside, kaempferol, hexoside, luteolin hexoside, isovitexin,20 vitexin,23,19 naringenin-7-neohesperidoside, diosmetin-7-neohesperidoside, chrysoeriol dihexoside, chrysoeriol-7-β-D-glucoside, (+)-eriodictyol,21 luteolin,22,23 hesperitin,24 chrysoriol,25 tricin-7-glucoside, kaempferol-3-O-2″-(6‴-p-coumaroyl) glucosylrhamnoside, feruloylsaponarin, quercetin-3-O-β-D-(6″-O-(E)-p-coumaryl) glucopyranoside, kaempferol-3-O-(6″-O-p-coumaroyl) glucoside (potengriffioside A) |
Phenolic acids/phenolic acid derivatives | caffeoyl-hexose, coumaryl-hexose isomers, caffeoyl-fructofuranosyl-glucopyranoside or 6-O-caffeoylsophorose, feruloylsucrose isomers, ferulic acid hexoside, (trans)caffeoylshikimic acid isomers, rosmarinic acid,26 4-caffeoyl-5-feruloylquinic acid |
Terpenoid derivatives | blumenol-C-9-O-β-(6′-O-rhamnosyl-2′-O-β-glucuronosylglucoside), euphopubescenol,27 Taxchinin J, masilinic acid28 or corosolic acid,29 humilinolide C |
Iridoid derivatives | catalposide isomers, volvaltrate C, (+)-syringaresinol β-D-glucoside |
Coumarin derivatives | cantabilin, 7-methoxycoumarin30 |
Amino acid and Amino acid-sugar derivatives | tryptophan, glutamic acid,31 tyrosine, leucine, and phenylalanine, isoleucine, fructosyl-valine, glutimic acid hexose, fructosyl-tyrosine, fructosyl-leucine, fructosyl-tryptophan, phenylalanine methyl ester,32 5-hydroxytryptophane |
Nucleoside derivatives | oxiamin, guanosine, adenosine |
Others | trihydroxystearic acid, trihydroxy-10-transoctadecenoic acid, trihydroxy-10,15-octadecadienoate I & II, linoleic acid 13-hydroperoxide I & II, linolenic acid monoglyceride, kahweol palmitate,33 α-linolenic acid,34,35 triglochinin isomers, [6]-shogaol,36 gingerglycolipid A, dihydrocapsiate,37 Vitamin B4, 2,6-tert-butylphenol |
| El-Desouky12 (ethyl acetate fraction) | |
Alkaloid | (S)-3,4,5-trihydroxy-1 H-pyrrol-2(5H)-one12 |
Phenolic Acid | caffeic acid38 |
Flavonoid/Flavonoid derivative | isoorientin,18 luteolin,22,23 vicenin 11, and 3,6,8-trimethoxy, 5,7,3',4'-tetrahydroxy flavone12 |
| Afifi13 (ethanolic/ether extract) | |
Flavonoid/flavonoid derivative | isoorientin,30,40 vitexin41 |
| Cole14 (Water extract) | |
Phenolic aldehyde | isovanillin14 |
Fatty acids | linolenic acid14 |
Phytosterol | β-sitosterol14 |
aBoldface text indicates anticancer action for individual constituents or constituents studied in combination with other phytochemicals found in Arum palaestinum.
Anticarcinogenic actions of fortified Arum palaestinum extract, in vitro and in vivo
Much of the most interesting work to date exploring the anticarcinogenic properties of Arum palaestinum has been done with an extract fortified with the already naturally occurring constituents of isovanillin, linolenic acid, and β-sitosterol.14 The in vitro and in vivo work published with this fortified extract, known as GZ17, are reviewed below.
In vitro
Cells from the prostate cancer cell line 22Rv1, a recognized model of androgen-independent cancer,42 were placed in a 3D cell culture system forming spheroids. These cells were then exposed to increasing doses of unfortified extract of Arum palaestinum, the fortified extract GZ17, the additives used for fortification by themselves, or control vehicle, at doses ranging from 0 mg/mL to 6.25 mg/mL, for 24 hours prior to assessment of cell number by fluorescent assay. As shown in Figure 4, GZ17 shows significant reduction of prostate cancer cell number at a concentration of about 0.2 mg/mL, compared to control vehicle. Moreover, at concentrations of 3.13 mg/mL and 6.25 mg/mL the inhibitory effect of GZ17 is significantly greater than that of usual water extract of Arum palaestinum. Most interestingly, the additives themselves used to fortify the extract show no significantly differing effects compared to the unfortified extract of Arum palaestinum. Thus, the fortified extract shows increased inhibitory effects on prostate cancer cells not seen by its individual parts, an example of synergy.
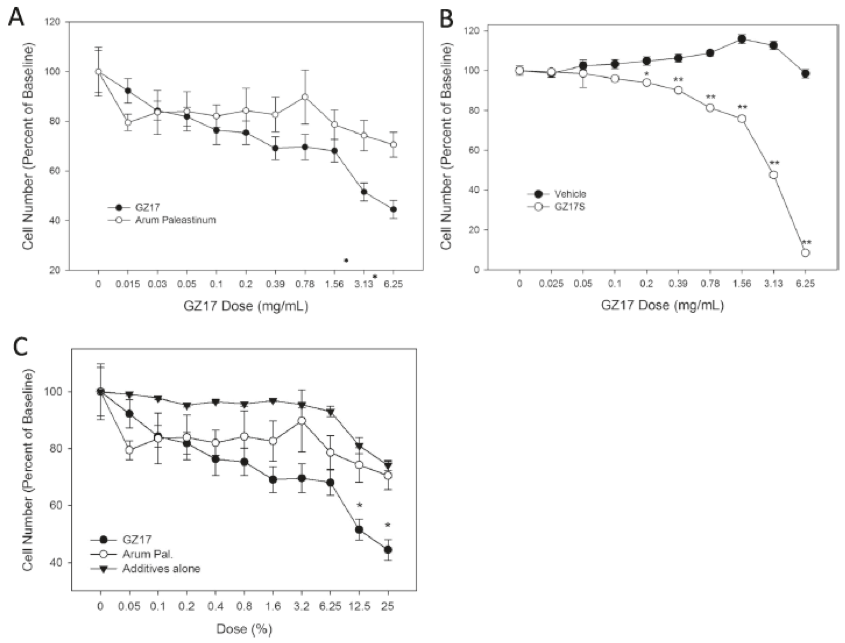
Figure 4. Effects of GZ17 on prostate cell number. A, Prostate cancer cells respond to Arum palaestinium and fortified GZ17 in a dose-dependent manner. B, GZ17 is more potent at inducing cell death, compared to vehicle. C, Individual components of GZ17 tested alone have little effect on cell viability.
Figure reproduced with permission from Genzada Pharmaceuticals.14
In vivo
Prostate cancer cells of line PC3-MM2 were injected subcutaneously into male nude mice and the mice were subsequently followed over a 3-week period. Mice received either the vehicle (purified water) or GZ17 by oral gavage at a dose of 1,000 mg/kg. Tumor sizes were measured twice per week with calipers. Main findings of this study were as follows:
- Prostate tumors grew steadily throughout the 3-week treatment period in the vehicle-treated mice.
- The size of tumors and the rate of tumor growth were both statistically significantly greater in the mice treated with vehicle vs the mice treated with GZ17.
- There was a 122% increase in tumor size by the end of the study in the vehicle-treated animals (Figure 5).
- GZ17 slowed tumor growth when compared to the vehicle group with only a 9.6% increase in tumor size.
- By day 6 and for all subsequent measurements there was a statistically significant increase in the tumor volume of the vehicle-treated mice. There was no statistically significant increase in tumor size until day 16 in the GZ17-treated group.
- The rate of tumor volume increase was 73 mm3/day in the vehicle group and 24 mm3/day in the GZ17-treated mice.
- Tumor growth delay was measured when control tumors reached a volume of 500 mm3, which occurred on day 7.1 in the control animals and day 16.3 in the GZ17-treated mice.
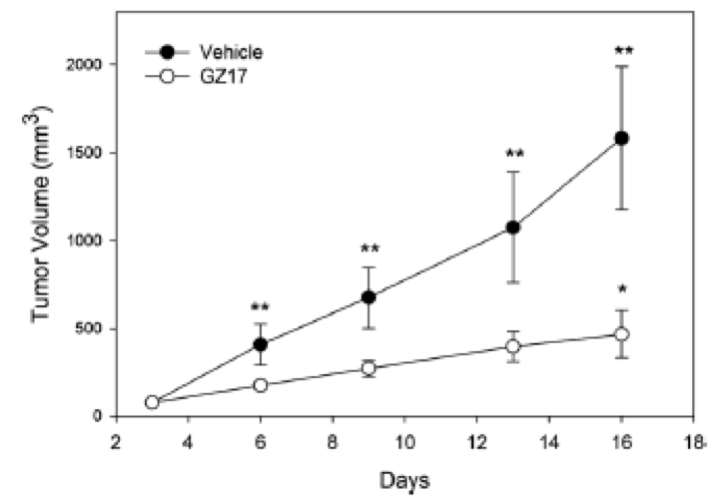
Figure 5. Tumor growth in vehicle-treated mice and mice treated with CZ17.
Figure reproduced with permission from Genzada Pharmaceuticals.14
Mechanism of action: increase of caspase-6
Caspase-6 is a known pro-apoptotic protein whose expression in prostate intraepithelial tissue is significantly increased in precancerous and frankly cancerous tissue compared to normal prostate glandular tissue.43 Additionally, the pro-apoptotic effect of caspase-6 is also associated with the anticancer effects of both natural and drug products in multiple other solid tumor cell lines.44,45
As shown in Figure 6, prostate cancer cells (line 22 RV1) exposed to increasing concentrations of GZ17 for 24 hours showed increased caspase-6 activity at a concentration of 0.1 mg/mL; cell death was noted when the concentration of GZ17 was increased to 0.2 mg/mL.
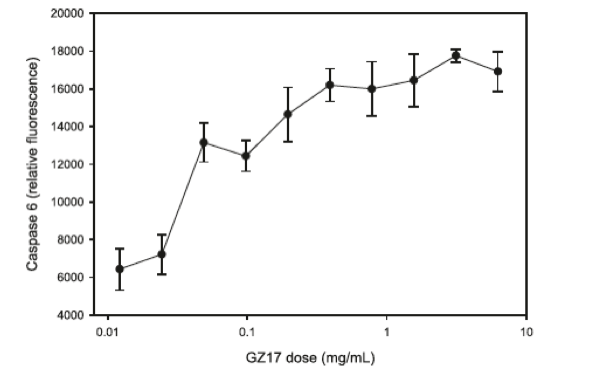
Figure 6. Effects of GZ17 on caspase-6 activity.
Figure reproduced with permission from Genzada Pharmaceuticals.14
Thus, the measurable anticarcinogenic effects of the fortified extract of Arum palaestinum are accompanied by an understood mechanism of action that could apply across multiple types of solid tumors.
In vitro and animal safety data on fortified extract, as well as historical perspective on Arum palaestinum generally
Cole14 examined the toxicity of GZ17 against normal human islet cells and prostate cancer cells and found the dose-dependent toxicity of GZ17 was not mirrored in healthy islet cells, as shown in Figure 7A. Moreover, the half-maximal inhibitory concentration (IC50) of GZ17 against prostate cancer cells was appreciably lower than that of the IC50 for vascular smooth muscle cells and fibroblasts, as shown in Figure 7B.
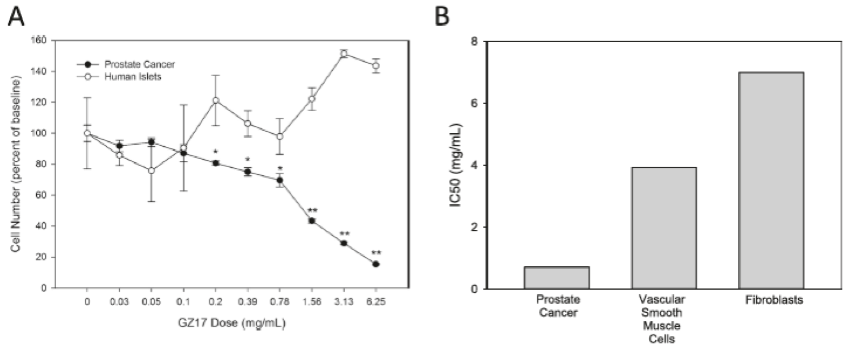
Figure 7. Relative toxicity of GZ17 against prostate cancer cells and normal cells. A, GZ17 is less toxic on normal human islet cells. B, Half maximal inhibitory concentration (IC50) of GZ17 against prostate cancer cells is lower than vascular smooth muscle cells and human fibroblasts.
Figure reproduced with permission from Genzada Pharmaceuticals.14
Acute toxicity testing of the fortified extract, GZ17, has been accomplished by testing 5,000 mg/kg of the extract delivered by oral gavage to 3 female Sprague-Dawley rats.14 The rats experienced no lethality and showed no signs of toxicity, while continuing to exhibit weight gain over the course of the study (Table 2). At necropsy, all abdominal/thoracic organs appeared normal.
Table 2. Toxicity of GZ17 in Rats
| Rat number | Initial Weight (gm) | Day 7 weight (gm) | Completion weight (gm) | % weight gain |
| 1 | 170 | 193 | 239 | 71 |
| 2 | 200 | 219 | 248 | 81 |
| 3 | 176 | 194 | 240 | 73 |
Table reproduced with permission from Genzada Pharmaceuticals.14
In addition to the acute toxicity work done in rats, the mice used in the in vivo 3-week experiment who showed attenuation of tumor growth were also assessed for potential changes in organ weight or morphology, compared to mice treated with vehicle (controls). As shown below in Table 3 and Figure 8, there was no difference seen in the organ weight or morphology across the 2 groups, and the weight of the treatment mice was not significantly less than the control mice.
Table 3. Comparison of Organ Weight in Mice Treated with GZ17 vs Vehicle
| Tissue | Vehicle | GZ17 | P |
| Brain | 0.40±0.03 | 0.40±0.04 | 0.94 |
| Heart | 0.18±0.01 | 0.19±0.01 | 0.35 |
| Kidney | 0.44±0.03 | 0.46±0.02 | 0.59 |
| Liver | 1.41±0.08 | 1.40±0.10 | 0.86 |
| Lung | 0.22±0.02 | 0.28±0.03 | 0.16 |
| Spleen | 0.09±0.01 | 0.09±0.01 | 0.57 |
Table reproduced with permission from Genzada Pharmaceuticals.14
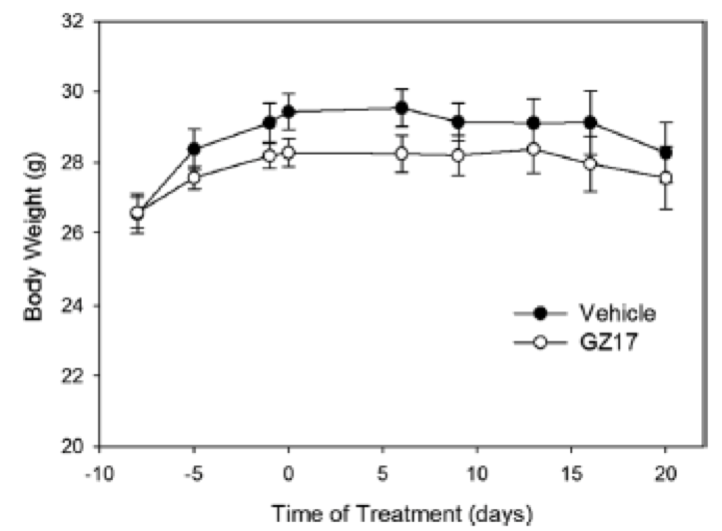
Figure 8. Changes in body weight in mice treated with GZ17 vs vehicle.
Figure reproduced with permission from Genzada Pharmaceuticals.14
Since ancient times, preparation of the leaves of Arum palaestinum as a food has included boiling the leaves in water and decanting the water several times to remove high levels of oxalates.11
Though in a Middle Eastern population safety concerns were identified with a number of herbal preparations used as CAM, no concerns of negative herb-drug interactions or direct toxic effects were associated with Arum palaestinum, upon review of available literature.46
Discussion
As reviewed above, Arum palaestinum has extensive historical use as a food-medicine, with one of the most extensive traditional uses being as an herbal medicine used to treat cancer. When individual chemical constituents from the major chemical categories of Arum palaestinum are surveyed and connected to published literature, it is seen that a substantial number of the phytochemicals in Arum palaestinum show anticarcinogenic activity in their own right. Moreover, an extract of Arum palaestinum fortified with isovanillin, linolenic acid, and β-sitosterol shows very promising action against prostate cancer cells in vitro and in a mouse model. The anticancer potential of Arum palaestinum, combined with other emerging areas of therapeutic interest, such as preliminary evidence suggesting potential antinociceptive properties,47 portends an exciting future in the research of this botanical with an already rich ethnobotanical past.
Conclusions
Arum palaestinum is a botanical with extensive use within Traditional Arabic Palestinian herbal medicine, as well as in other regions of the Mediterranean. It contains a number of phytochemicals known to exert anticarcinogenic effects, and when its water extract is fortified with several constituents endemic to the plant, namely isovanillin, linolenic acid, and β-sitosterol, a synergistic anticarcinogenic effect is seen against prostate cancer cells. Future human clinical trials are warranted and needed to establish best parameters of use and to understand if extracts of Arum palaestinum may prove useful in the treatment of human carcinomas.
Disclosure Statement
The author is an independent contractor who was compensated by Genzada Pharmaceuticals for the completion of the review and preparation of the manuscript.






