Abstract
Two recent studies on the effects of chocolate consumption and incidence of heart failure suggest that consuming increasing amounts of chocolate is associated with declining risk of hospitalization or mortality from heart failure. Yet this effect levels off as consumption increases and may even decline with high chocolate consumption. These associations between risk and consumption can be described by a U-shaped graph. This graphic pattern is not unique to chocolate but also may be used to describe a wide array of natural supplements and their impact on health. For chocolate and an increasing number of other natural substances it appears that there is an optimal level of consumption that provides maximal health benefit and that more or less is associated with reduced benefit. This paper reviews some of these supplements and the current information suggesting ideal dosing.
Introduction
A common assumption when examining biologic phenomena is that cause and effect should display a linear relationship: As a causative stimulus increases, the measured effect it has will increase in direct proportion. When this is true, a dose response is represented graphically by a straight line with a constant slope.
Real life is rarely so simple. The slope of the graph may vary; certain strength “doses” of stimuli may have greater or lesser effect. Many biological effects actually display not just differing effects based on dose strength, but even opposite effects. When graphed, these actions are represented by U-shaped or J-shaped graphs rather than straight lines. This is not uncommon when it comes to nutritional supplements and lifestyle interventions; certain doses produce the desired effects, but larger or smaller doses may produce less benefit or even negative effects. I refer to these ideal dose ranges as the “sweet spot” in the curve.
Chocolate and Heart Health
Steinhaus et al published a paper in the January 2017 issue of the American Heart Journal. The authors conducted a prospective cohort study using data from 31,917 men, 45 to 79 years old, who had participated in the “Cohort of Swedish Men” study. None of the men had a history of myocardial infarction, diabetes, or heart failure at baseline. The participants were followed for heart failure hospitalization or mortality from January 1, 1998 to December 31, 2011, using record linkage to the Swedish inpatient and cause-of-death registries, allowing the researchers to track hospitalizations or death of participants from heart failure. Participants’ chocolate consumption was assessed through self-administered food frequency questionnaires.
Hormesis is a term toxicologists use to describe a J-shaped or U-shaped dose response characterized by a beneficial effect at low doses and a toxic (or inhibitory or opposite) effect at higher doses.
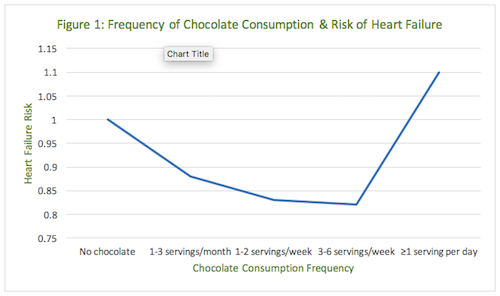
During 14 years of follow-up, 2,157 of the men in the cohort were hospitalized (n=1,901) or died from incident heart failure (n=256). Compared with subjects who reported no chocolate intake, the multivariable-adjusted rate ratio of heart failure was 0.88 (95% confidence interval [CI]: 0.78-0.99) for those consuming 1 to 3 servings per month; 0.83 (95% CI: 0.72-0.94) for those consuming 1 to 2 servings per week; 0.82 (95% CI: 0.68-0.99) for those consuming 3 to 6 servings per week; and 1.10 (95% CI: 0.84-1.45) for those consuming ≥1 serving per day (P-value for quadratic trend=0.001).1 (Table 1)
| Chocolate consumption | Heart failure risk |
| No chocolate | 1.00 |
| 1-3 servings/month | 0.88 |
| 1-2 servings/week | 0.83 |
| 3-6 servings/week | 0.82 |
| >1 serving per day | 1.10 (not significant) |
At first glance the implications of this large, prospective cohort study seem simple and obvious. Eating chocolate may be good for men because it is associated with decreasing risks of heart failure. Moderate chocolate consumption was associated with a lower rate of hospitalization or death from heart failure, but the protective association was not observed among individuals who consumed ≥1 serving per day. If one plots a graph of these results, with risk of heart failure on the y-axis and frequency of chocolate consumption on the x-axis, it creates a J-shaped curve (Figure 1).
In an earlier study published in 2010, these same authors reported a very similar benefit in women who consumed moderate amounts of chocolate. Women who consumed 1 to 3 servings of chocolate per month had a 26% lower risk of heart failure than women with no regular chocolate intake, and those who consumed 1 to 2 servings per week had a 32% decrease in risk. Risk may increase at higher levels of consumption, but the numbers did not reach statistical significance.2 These results are also a J-shaped curve.
A similar J-shaped relationship between dark chocolate consumption and serum C-reactive protein (CRP) was reported in 2008; individuals who consumed up to 1 serving (20 g) of dark chocolate every 3 days had serum CRP concentrations that were significantly lower than both nonconsumers and higher consumers. Since regular consumption of dark chocolate is associated with low serum concentrations of CRP, it is not surprising that these decreased levels of inflammation appear to translate into differing risk for heart failure.3 Again, chocolate consumption followed a J-shaped curve, suggesting more is not necessarily better.
These findings of benefit from chocolate are consistent with other past studies that suggest chocolate has beneficial effects on cardiovascular health.4-9 Multiple studies have reported both acute and chronic chocolate consumption reduce systolic and diastolic blood pressure.10-14 In addition, habitual chocolate eating is associated with lower incidence of stroke and myocardial infarction (MI),15 lower incidence of mortality from coronary heart disease,5 lower cardiac mortality and heart failure after incident MI,16 and improved vascular function in patients with heart failure.17 Thus these current Steinhaus findings are consistent with earlier data and should come as no surprise.
The J-shaped dose response is worth investigating. Such dose-response curves draw our attention as they are not uncommon when describing biological responses to natural substances. J-shaped or U-shaped curves are classically associated with what are called hormetic responses. Hormesis is a term toxicologists use to describe a J-shaped or U-shaped dose response characterized by a beneficial effect at low doses and a toxic (or inhibitory or opposite) effect at higher doses.18
Forget the assumption that dose relationships should follow straight lines and instead start assuming that, in nature, J-shaped curves may actually be more common than we thought; the very nature of the response may change with the dose even to the degree of having the opposite effect at high doses of what occurred at low doses.
A 2010 prospective study (N=1.46 million) reported a J-shaped association between body mass index (BMI) and all-cause mortality after adjusting for potential confounders, including smoking and alcohol intake. All-cause mortality was lowest among those with BMI of 20.0 to 24.9 (kg/m2) and higher on either side of that interval.19 Thus, being either underweight or overweight increased one’s risk of death.
Vitamin D
Too little or too much vitamin D may also pose a problem. This should give us pause as many of us have practiced for years under the belief that high-normal levels of vitamin D posed no health risks to our patients. An October 2016 analysis of the European Prospective Investigation of Cancer and Nutrition (EPIC)-Norfolk Cohort reported, “In older adults, the relationship between vitamin D status and fracture risk was observed to be J-shaped.”20
Schwartz had reported back in 2014 on the Safety and Efficacy Large-scale Evaluation of COX-inhibiting Therapies (SELECT) trial data related to prostate cancer prevention that the effects of vitamin D followed a U-shaped curve. The authors noted that the ideal range of circulating vitamin D for preventing prostate cancer may be narrow, and that supplementing in men with adequate levels may be harmful. “There were U-shaped associations of vitamin D with total cancer risk: compared with the first quintile, HRs [hazard ratios] were 0.83, 0.74, 0.86 (95% CI: 0.69-1.07; P=0.181), and 0.98, for the second through fifth quintiles, respectively,” they reported. In addition, men with moderate concentrations (approximately 45–70 nmol/L) [18- 28 ng/ml] had significantly reduced risks.21 This “sweet spot” is notably lower than most of us might guess.
Studies that have looked at vitamin D levels and cardiovascular disease or overall mortality also report J-shaped associations. A 2014 analysis reported that, as expected, mortality risk increases in those who are deficient but also increases at serum vitamin D levels above 125 nmol/L (about 50 ng/mL).22 Many labs report the normal range for vitamin D as extending up to 100 ng/mL so practitioners may rationalize keeping patients at the upper end under the assumption that more is better.
This J-shaped curve in mortality was reported a year earlier in 2013, in an analysis of the National Health and Nutrition Examination Survey (NHANES) data. The authors reported that the lowest risk of mortality, what they called “the nadir of risk,” was at 81 nmol/L (95% CI: 73-90 nmol/L) or 32.4 ng/mL.23
Fish Oil
A November 2016 meta-analysis suggests that fish oil’s impact on depression also follows a J-shaped pattern. Data from 31 studies were pooled (N=255,076), yielding over 20,000 cases of depression. Fish consumption was associated with a 22% reduction in relative risk (RR) of depression (RR: 0.7; 95% CI: 0.69-0.89). Examination of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) supplement use revealed a J-shaped association, with a peak decreased risk at 1.8 g/d intake of n-3 polyunsaturated fatty acids (PUFA) (RR: 0.30; 95% CI: 0.09-0.98).24 Thus with fish oil it appears a moderate dose may offer greater benefit than higher or lower doses.
A 2010 study suggested a J-shaped curve for fish consumption for reducing psychotic-like symptoms. The associations were J-shaped with the strongest reduced risk for an intermediate intake of fish or PUFA. For fatty fish (herring/mackerel, salmon-type fish), the strongest inverse association was found for an intermediate intake (RR: 0.81; 95% CI: 0.66-0.98), whereas a high intake of fatty fish was associated with an increased risk of psychotic-like symptoms (RR: 1.90; 95% CI: 1.34-2.70).25
Vitamin C and Breast Cancer
There is reason to believe that vitamin C from the diet affects postmenopausal risk of breast cancer and this too has a sweet spot. A paper published in July 2016 reports on data from the E3N cohort in France, a group of 57,403 women followed for 13 years during which 2,482 cases of invasive breast cancer occurred. Dietary vitamin C intake was assessed via regular questionnaires during the study.
Vitamin C supplement use was associated with higher breast cancer risk in postmenopausal women in the fourth quartile of vitamin C intake from foods (hazard ratio (HR): 1.32; 95% CI: 1.04-1.67). This suggests a potential U- or J-shaped relation between total vitamin C intake and postmenopausal breast cancer risk. This could suggest menopausal women might be better off not taking vitamin C supplements if they have a healthy diet.26
Iodine
We’ve long known that too little or too much iodine is a problem clinically. In pregnancy this narrow range of safety is even more critical. A 2015 paper (N=7,190) of pregnant women in China states, “The upper limit of iodine intake during early pregnancy in an iodine-sufficient region should not exceed UIC 250 μg/L, because this is associated with a significantly high risk of subclinical hypothyroidism.”27
This iodine information is specifically for those who are iodine-deficient at the time of pregnancy. The US Institute of Medicine recommends much more than 250 µg during pregnancy. Suddenly dosing iodine to anyone who is deficient can induce hypothyroid response. Obviously dosing nutrients is more complicated than making any broad recommendations. Optimal dosing is complicated by many factors, not least of which is the individuality of one’s biochemical needs.
Discussion
The findings in these studies may result from confounders that are either not identified or overly compensated for. For instance, the vitamin D studies are observation studies, and they don’t comment on the vitamin D source. We may postulate that fish or dairy are the biggest sources in northern countries. To obtain high blood levels of vitamin D from fish, there is probably a larger intake of potentially toxic compounds such as mercury, polychlorinated biphenyls (PCBs), and others. Since these were not evaluated, we cannot exclude an effect from these to explain the J-shaped relationship. The same can be said for most observational studies.
In the case of chocolate, chocolate per se may be a health food but the behavior of someone eating more that 20 g per day can be problematic. So the U-shaped association may not be due to the chocolate intake but to other factors, such as overall diet.
Conclusion
While we might suspect that many other substances will also have a dosing “sweet spot,” at this time that ideal dose remains unknown for most substances. Based on reports of hormetic actions in vitro, we may eventually be able to define sweet spots for resveratrol,19 curcumin,28 berberine,29 green tea,30 licorice,31 olive oil polyphenols,32 and even blueberries.33 Until then we have to let go of the simplistic idea that if a little bit helps, more is always better. While our minds may reflexively conclude that larger doses and stronger concentrations will be more efficacious when treating patients with vitamins, supplements, or food, we need to be reminding ourselves that much of what we employ therapeutically has a sweet spot and that giving a surplus may reduce efficacy of treatment.
This limiting of doses may not always be the easiest thing to do; it may be hardest with chocolate.






