This is part of the October 2016 Special Issue on Immunology.
Abstract
A healthy and intact immune response requires coordination between skin, mucosal barriers, and both the innate and adaptive aspects of immune response. With an overarching mandate of protection, the blueprints of individual immune surveillance systems are inherited through family history and fashioned through interactions with the environment, including lifestyle choices and chemical exposures. The goal of this article is to provide an overview of the immune response and opportunities for assessment, treatment, and management from an integrative medical perspective.
Introduction
In biology immunity is the “ability of an organism to resist disease, either through the activities of specialized blood cells or antibodies produced by them in response to natural exposure or inoculation (active immunity) or by the injection of antiserum or the transfer of antibodies from a mother to her baby via the placenta or breast milk (passive immunity).”1 Our immune system must continually balance a state of readiness, having all the necessary biological responses prepared to defend us from infection, disease, or invasion of organisms while simultaneously maintaining a state of tolerance or trained immunity.2 Ideally, vigilance is tempered by tolerance as the immune system needs to avoid mounting attacks on self (ie, autoimmune diseases), reprogramming recognition molecules (ie, cancers cells), and overreacting with exposure to food and environmental antigens (ie, allergies and sensitivities).3,4 Clinical management includes an understanding of the functional aspects of the immune system and development of treatment plans that include targeted therapies to modulate immunity. Many of the treatment modalities help support the pillars of our immune system, which include barrier function as well as the innate and adaptive responses.
Overview of Immune System Structure and Function
The immune system comprises a complex network of organs, vessels, cells, and proteins. The skin and mucosal surfaces create a mechanical and functional barrier5 to protect against and eliminate environmental debris and foreign invaders. For example, in the lungs mucus and the mucociliary “escalator” system work together with columnar epithelium to mechanically whisk microbes upward and outward. Columnar epithelial cells also secrete cytokines and host-defense molecules (human β-defensins, lysozyme, lactoferrin, cathelicidin LL-37, and surfactant proteins A and D) resulting in near-sterile airways when fully functional.6 Bodily secretions (eg, saliva, stomach acid, and tears) also combine with biological reflexes such as coughing, sneezing, vomiting, and diarrhea in an attempt to eliminate potentially dangerous organisms or irritants (antigens).
The bone marrow, thymus, and lymphocytes are the primary lymph organs; secondary organs include the lymph nodes, spleen, mucosal membranes, and the intestinal gut-associated lymphoid tissue (GALT), which includes the tonsils, Peyer's patches, and immune aggregates of the esophagus, stomach, and lamina propria. Together these organs function and orchestrate the maturation of immune cells and provide access to the non-immune privileged (eyes, placenta, fetus, testes, and central nervous system) systemic circulatory environment.7 In addition, normal human flora (the microbiota)8 work synergistically with skin and mucosal surfaces to maintain the functional barrier and create as healthy an ecosystem as possible. Although the exact distribution, content, phenotype, and genotype of prebiotics and probiotics have yet to be fully understood, it is clear that significant disruption of the microbiota modulates gut, brain, and immune function. For example, recent research suggests that short-term restriction of short-chain fermentable carbohydrates (the low-fermentable oligosaccharides, disaccharides, monosaccharides, and polyols [FODMAP] diet),9 which directly impacts the microbiota, may be clinically useful. Additionally, prebiotics, probiotics, and synbiotics may have the greatest health effects when they are incorporated into the diet early in life.10
Cellular Components and Innate and Adaptive Arms
All immune cells are derived from hematopoietic stem cells found in the bone marrow. Stem cells give rise to both the mature myeloid cells (red blood cells [RBCs], platelets, neutrophils, eosinophils, basophils, and mast cells) and lymphoid (natural killer [NK], B, and T) cells, key players in the innate and adaptive immune responses. Although traditionally these were distinct arms, many overlapping and intersecting functions of the humoral or cell-mediated response are now being discovered.
However, we can still define the role of each arm with some distinction. The innate immune system is fast-acting (~0-15hrs) and composed of epithelial barriers, phagocytic granulocytes, dendritic, and NK cells as well as plasma proteins of the complement cascade (C1q, C2, C3, C3c, C4, and C4d). The overarching clinical symptoms of classic inflammation (calor, dolor, rubor, tumor)11 are indicative of the innate response. The responding innate immune cells have the unique capacity to recognize and respond to evolutionarily conserved patterns upon exposure to foreign stimuli, therefore they act immediately. Pattern-recognition receptors (PRRs) embedded on macrophages and dendritic cells can detect or read patterns found in components of bacterial and fungal cell walls and viral nucleic acids.12 These PRRs include Toll-like and C-type lectin receptors. Pattern recognition receptors are reinforced by the complement cascade. The complement cascade is a complex of proteins produced by the liver and activated in plasma. The complement cascade modulates the systemic responses (inflammation, anaphylaxis) and is now believed to bridge and inform both innate and adaptive immunity.13,14
When bacteria, viruses, or allergens (antigens collectively) evade innate responses, the adaptive system is engaged. The adaptive arm of the immune system is delayed (up to 5 days) and consists of critical antigen presenting cells (APCs), which orchestrate responses; T and B lymphocytes; plasma cells; and the antigen-specific antibodies they produce. Dendritic cells are considered the primary “professional APCs” in addition to macrophages and nonprofessional APCs, such as B cells and epithelial cells.15,16 Antigen-presenting cells are responsible for antigen engulfment, uptake, processing, and presentation to the adaptive cells through engagement with receptor cells such as the T cell receptor (TCR). Once antigen-rich APCs engage T helper (Th) cells via their TCRs, the Th0 cells (precursors to all Th types) activate, proliferate, and expand within a skewed inflammatory milieu. T helper 1 (Th1) cells produce interferon gamma (IFN-?), interleukin-2 (IL-2), and granulocyte-macrophage colony-stimulating factor (GM-CSF) and modulate cell-mediated responses. Classically, Th2 cells produce IL-4, IL-5, IL-6, IL-9, IL-13, and IL-25 and drive the IgE-mediated antiparasite, antiallergy, and antibody responses commonly seen in clinic.17,18 Regulatory T cells (Tregs) with a cell phenotype of CD4+CD25+FoxP3+ produce IL-10 and transforming growth factor (TGF)-b and are intimately involved in chronic inflammation, scar formation, and the control of other cell types. There are numerous other sub lineages (Th17, Th22, Th9) being evaluated in the scientific literature that are generated by variations in the degree of antigen receptor engagement, the robustness of the secondary cytokine and chemokine signals, and the inflammatory cascade. The ability of dendritic cells to orchestrate immune responses makes them novel therapeutic targets in allergy, autoimmunity, and cancer.19,20
Numerous integrative therapies, dietary supplements, and botanicals (and their extracts) are being investigated for their ability to affect dendritic and Th cells, their activation status, and their ability to modulate the cytokines and chemokines they produce. For example, previous work in my laboratory in preclinical models has demonstrated that the pineapple extract bromelain is a potent antiallergy and asthma therapy. Our group has demonstrated in a mouse model that bromelain lessens immune response to an antigenic stimulus.21 Specifically, bromelain ingestion led to significant inhibition of allergic sensitization (egg antigen) via direct modulation of dendritic cells (CD11c+) as determined by reduced ovalbumin-specific IgE, antigen-specific CD8 T cells, and CD44 (a critical T cell receptor involved in allergic immune response).22,23 Ongoing studies will determine bromelain’s optimal dose in allergies and asthma in humans. Other groups are also actively evaluating how dendritic cells can be affected by natural products24,25 such as vitamin D26,27 and probiotics,28 and what clinical impact these findings will have. When possible all treatment modalities should be evaluated in the context of patient care and backed up with objective diagnostics and laboratory measures.
Laboratory Measurement of Immune System and Inflammatory Response
Laboratory evaluation and determination of the degree of immune system dysregulation and the contribution of inflammatory responses can be complex and overwhelming. A systematic approach based on the family and personal history (work and environmental exposure, sexual history, prescription and recreational drug use), review of symptoms (ROS), and physical exam findings is fundamental. Detailed personal and family history may also reveal multiple chronic immune-mediated conditions or conditions that follow a distinct inheritance pattern (for example, through males on the maternal side). In pediatric patients, the ROS may indicate delayed growth, failure to thrive, malabsorption, or developmental issues. In adults, common underlying symptoms may include frequent, recurrent colds and flus, less common infections, iron-resistant anemias, severe eczema, allergies, and hyper-IgE or allergic eosinophilic syndromes. Collecting a detailed history (classic homeopathic interview) will reveal the cause much of the time. These are the patients who present with “textbook symptoms” that will lead directly to laboratory testing that confirms the diagnosis, such as anemia revealed by a complete blood count (CBC)/iron panel. However, clinical presentations often are not straightforward and labs are unremarkable. Recently I had a patient present with a rash, mild irritable bowel syndrome, difficulty concentrating, and fatigue. Although my first thoughts were food-related allergy or atopy, all allergy tests were negative; testing eventually revealed a genetically altered uridine diphosphate glucuronosyltransferase enzyme (UGT1A1) and the subsequent diagnosis of Gilbert’s syndrome. Therefore, a stepwise diagnostic approach coupled with patient feedback throughout clinical interventions is warranted.
Table 1 outlines several options to consider when embarking on a standard laboratory evaluation of inflammatory and immune-mediated conditions. Even when immune dysregulation is not the chief complaint, several of these lab findings may be positive in patients who present with common symptoms such as pain, fatigue, insomnia, depression, anxiety, gastrointestinal complaints, and stress response. All labs in Table 1 can be ordered from any local or regional Quest Diagnostics lab (or similar lab).
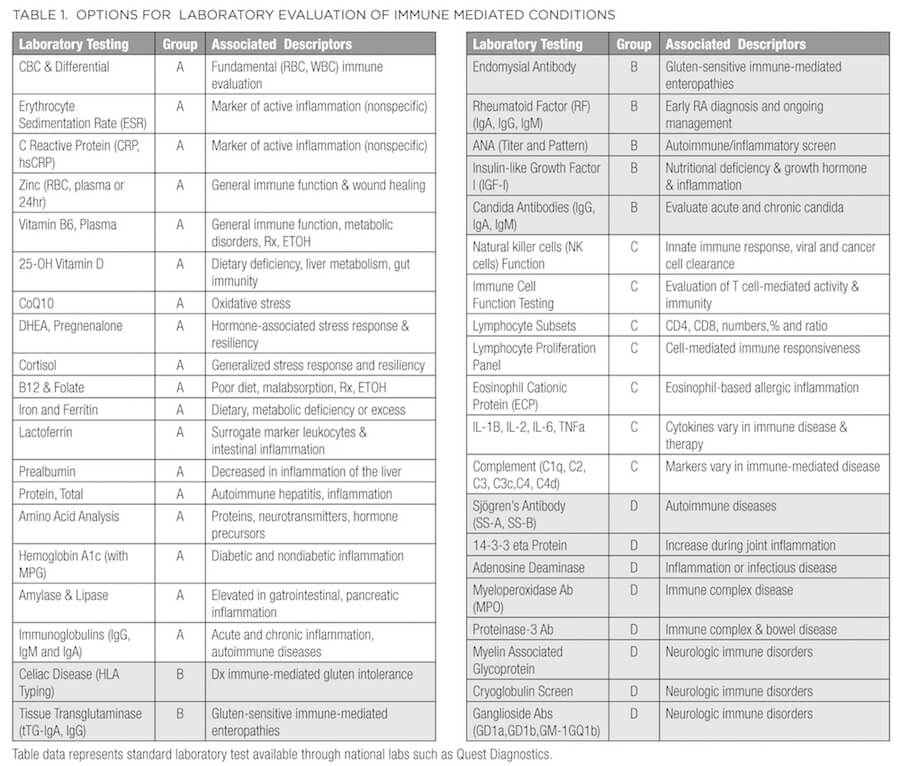
For ease of use and clinical application, several options for laboratory evaluation of common immune-mediated conditions are grouped from A (fundamental) through D (more advanced). This is not meant to be an exhaustive list but will provide context and reminders as you proceed with diagnosis and clinical management. Group A represents fundamental assessment beginning with a CBC to evaluate anemia. A helpful tool to remember when evaluating CBC results (sometimes referred to as the “rule of 3”) is that hemoglobin equals approximately 3 times the RBC count, and hematocrit equals approximately 3 times hemoglobin. For example, using typical values for an otherwise healthy adult man, if the RBC count is 5 x 106/uL, hemoglobin would be roughly 15 g/dL and hematocrit roughly 45%. When reviewing the white blood cell (WBC) count, consider both the total count and the differential (percentage of each type of WBC), and platelets. Variation in WBCs and their subtypes (either too low or high) can indicate immune compromise (low total numbers, lymphocytes, or neutrophils), allergic response (elevated eosinophils), autoimmune disorder, and cancer. The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels provide evidence of an active inflammatory process that may require further evaluation.29 Patients can also be evaluated for nutrition-related immune status by testing for levels of zinc, vitamin B6, 25-OH cholecalciferol, coenzyme Q10, vitamin B12/methylmalonic acid (MMA), folate, iron, ferritin, and protein (total, albumin, and globulin) and ordering an amino acid analysis. Any of these can be used in combination to determine if nutrient absorption and/or metabolism are issues. Systemic stress or resiliency can be determined with salivary or serum cortisol (morning and evening) and hormone cascades such as dehydroepiandrosterone (DHEA) and pregnenolone and their downstream products. The addition of hemoglobin A1c (with mean plasma glucose) will direct therapeutic goals toward sugar regulation, and amylase and lipase abnormalities may prompt a referral to a specialist in gastroenterology for further work-up.
Altered immunoglobulins30 (Igs) may suggest broad compromise in immunity or autoimmunity, B cell production (all Igs reduced), a specific cancer (eg, lymphoma, multiple myeloma), or an autoimmune (eg, rheumatoid arthritis, system lupus erythematosus) diagnosis. Labs that reveal an increase or decrease in selective Igs may suggest an active ongoing infection (IgM); gastrointestinal mucosal involvement (reduced IgA); chronic infection and immunocompromise (IgG increased); allergic, dermatitis, or parasitic activity; or hyper IgE syndrome (increased IgE). Patients who are responding to vaccination may see a 2- to 5-fold increase in immunoglobulin response (4-8 weeks primary; 8-12 weeks memory), so determine if the CBC, WBC differential, or humoral response is altered due to vaccination. Depending on the patient’s symptoms, further evaluation and referral to an appropriate specialist for a more comprehensive immunological evaluation of immune dysregulation may be warranted.29,30
Group B is directed toward immune-mediated gluten intolerance (celiac disease) and enteropathies as well as the initial screen for autoimmunity (testing for antinuclear antibodies [ANA]) and rheumatoid arthritis, metabolic inflammation (IGF-I) and concomitant immunologic response to Candida. If there is indication of immune dysregulation (low or high WBC subtypes), chronic unresolved infection, inflammation, or frequent colds and flus, Group C will help evaluate innate and adaptive arms of the immune response. These tests may identify and enumerate additional lymphocyte cell subpopulations based on flow cytometry and identification of cell surface receptors (such as CD4 and CD8 T cells), or they may be functional tests performed on isolated WBC subsets, which are cultured in vitro and challenged with a variety of antigenic and cellular stimuli. Tests include evaluation of innate, NK cell function and proliferation panels as well as selected proteins of the complement (C1q, C2, C3, C3c, C4, C4d) cascade and cytokine (IL-1B, IL-2, IL-6, tumor necrosis factor [TNF]) production. Based on test results you may decide to enhance innate and or humoral immunity through lifestyle modification or medicinally with dietary polysaccharides, larch arabinogalactan, Ganoderma, Terminalia bellirica, Salacia chinensis, Zingiber montanum, or Peltophorum pterocarpum.31-35
Group D provides a more detailed evaluation and diagnosis of selected autoimmune conditions such as Sjögren’s syndrome, arthritis (14-3-3 proteins), immune complex disease (myeloperoxidase), or neurologic immune disorders (myelin-associated glycoprotein antibody). Diagnosis of autoimmune conditions should be done in conjunction with an immunologist and every effort put forth to work together for the betterment of the patient.
Testing Allergies/Intolerances
In addition to the standard metabolic labs described above, patients presenting with conditions such as ongoing or seasonal sinusitis, chronic recurrent rhinitis, postnasal drip, sinus pressure, headaches, cough, allergic asthma, eczema, dermatitis, and psoriasis, or elevation of WBCs, subsets of eosinophils, and/or total IgE may benefit from an allergy and/or sensitivity panel. As summarized in Table 2, some of these panels vary based on the environmental allergens exposed by region. For example, Connecticut is designated as Region I, along with the rest of New England, New Jersey, New York, and Pennsylvania. Therefore, consider this regional variation before ordering. In addition to the traditional IgE-based allergy test profiles (Table 3), many laboratories now provide IgG-based panels (Table 4) to evaluate sensitivity and intolerance in addition to the anaphylactic or immediate type 1 IgE-driven immune hypersensitivity responses.36
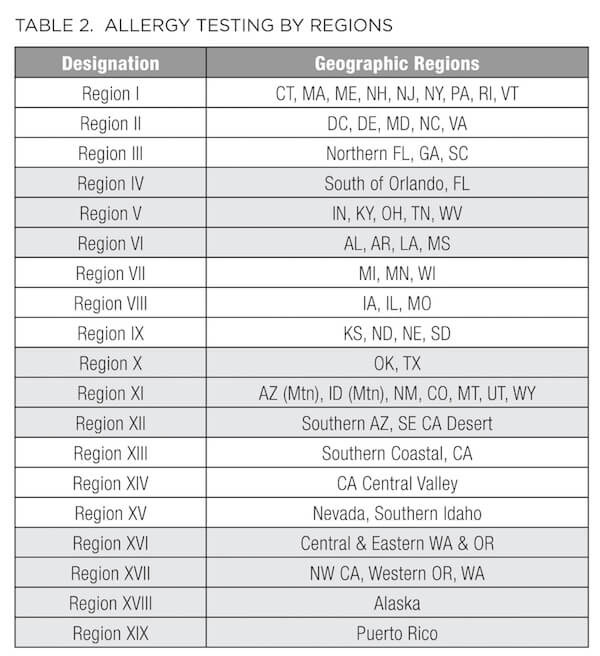
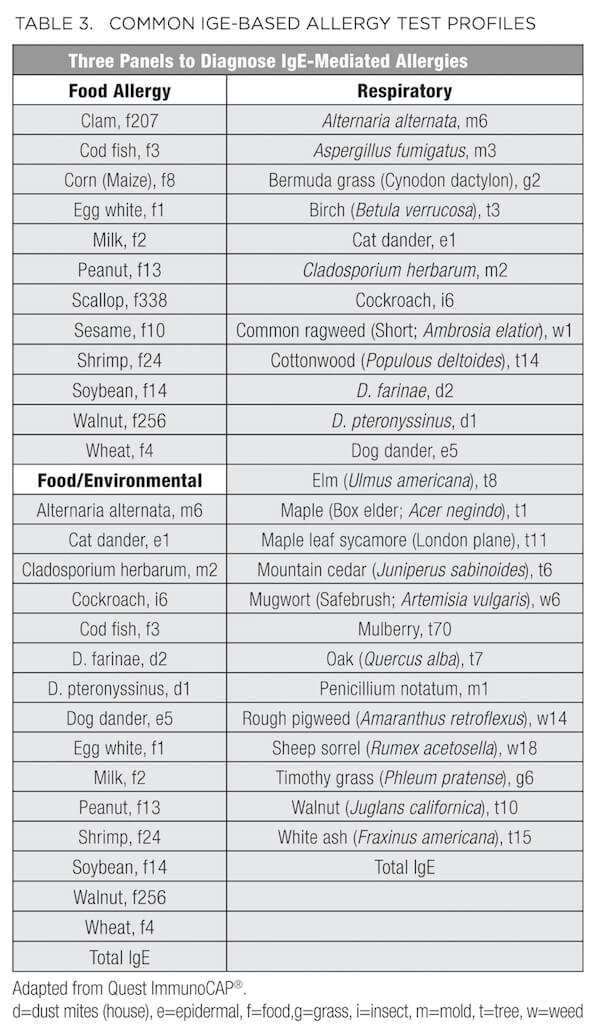
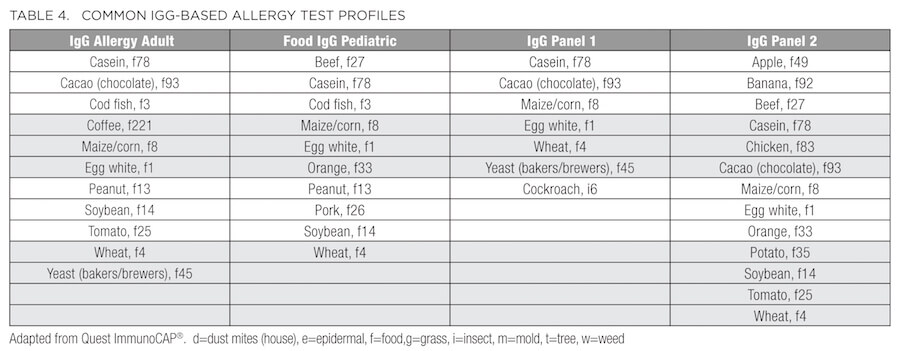
Diagnosing intolerances and allergies can be difficult because symptoms often overlap or may be confused with other conditions, such as gas, bloating, abdominal pain, diarrhea, constipation, heartburn, fatigue or loss of energy, headaches and migraines, anxiety, depression, mood swings, poor concentration, and muscle/joint pain. According to the guidelines set by the National Institute of Allergy and Infectious Diseases,37 food allergy arises from (and is reproduced by) a specific immune response on exposure to a given food. However, an intolerance may cause the same reproducible adverse reaction but may not have an established underlying immune-mediated mechanism. For example, someone who is truly allergic to cow's milk has an immune system response to milk protein (casein or whey) and therefore has a food allergy. But someone who has difficulty drinking milk due to an inability to digest lactose in milk has food intolerance, which may be due to low lactase production or any number of problems with the digestive system. Similarly, the diagnosis of celiac disease is based on a multigenic immune-mediated enteropathy triggered by dietary glutens, present in wheat, barley, and rye. With celiac disease, human leukocyte antigen (HLA) typing is performed; 90% of celiac patients express the HLA-DQ2 molecules. Gluten peptides presented by these HLA molecules induce an abnormal mucosal immune response and tissue damage.38
Conclusion and Future Directions
The immune system represents a complex network of organs, tissues, and blood products whose role is to balance a state of tolerance with swift and decisive action. As integrative practitioners our focus is to promote lifestyle balance and immune optimization by minimizing the impact of stressors and maximizing therapies that positively modulate the immune response. Fundamental tools include a comprehensive understanding of the immune system, its diagnosis, and its management; proper application of healthy diets, food elimination, and detoxification; exercise; dietary supplements; lifestyle interventions such as stress reduction, sleep, meditation, and energy therapies; and timely diagnosis and management of allergies, autoimmunity, cancer, and inflammatory processes. Communicating findings to both patients and referring doctors can provide novel treatment options for complex immune-mediated diseases that traditionally rely on steroids, immunosuppressants, and chemotherapy agents as first-line treatments. Keeping patients safe and caregivers engaged will ultimately create an environment in which integrative therapies can be used and given the time they need to turn around the most complex immune-mediated conditions.







