The current standard of care medical model is a disease-based approach. Diagnosis and determination of appropriate treatment options are complex processes that require a move toward a more patient-centric model of care that includes individualized diagnosis and treatment. The functional medicine model requires a diagnosis, but in addition, an understanding of the unique mechanisms including predisposing genetic factors, biochemical and psychosocial factors, and underlying dysfunctions in order to prescribe appropriate evidence-based therapies to improve overall health. The purpose of this case series is to describe the potential benefits of implementing a functional medicine approach for various chronic, complex pain syndromes.
Abstract
Background: The current standard of care medical model is a disease-based approach. Diagnosis and determination of appropriate treatment options are complex processes that require a move toward a more patient-centric model of care that includes individualized diagnosis and treatment. The functional medicine model requires a diagnosis, but in addition, an understanding of the unique mechanisms including predisposing genetic factors, biochemical and psychosocial factors, and underlying dysfunctions in order to prescribe appropriate evidence-based therapies to improve overall health. Objectives: The purpose of this case series is to describe the potential benefits of implementing a functional medicine approach for various chronic, complex pain syndromes. Methods: Three patients with different underlying diagnoses including chronic, neuropathic pain, radiculopathy, and paresthesia were assessed and treated using the functional medicine model. Outcome measures were obtained pre- and post-treatment. These measures included validated surveys (short-form McGill Pain Questionnaire, DN4 Questionnaire, Medical Symptoms Questionnaire, Fibromyalgia Impact Questionnaire, and the Brief Pain Inventory scale) and objective markers such as blood and salivary biomarkers of health and function. Results: Patients had clinically significant pain reduction and improved health and function as documented with both subjective and objective outcome measures up to as much as 18 months after treatment initiation. No serious adverse effects were reported. Conclusion: This unique case series suggests the functional medicine model may be of benefit in the assessment and management of patients with complex pain syndromes. Further investigations with randomized controlled trials in a more specific pain population may be warranted.
Introduction
A comprehensive assessment of multiple systems offers chronic pain patients a treatment strategy to restore the homeostatic balance that is required for alleviating chronic pain syndromes. Blocking individual downstream mediators with drugs may temporarily uncouple the pain process but often does not lead to long-term pain reduction and increased functionality. An evidence-based1 functional medicine model is aimed at diagnosing and treating multiple levels of causation and provides a more comprehensive approach to patient care.
Case Study 1: Facial Neuropathic Pain
In January 2012, a 70-year-old female presented with severe facial nerve pain after a facial cosmetic surgery more than 1 year previously. The pain was described as a sharp, stabbing pain and was localized over the distribution of the facial nerve. The patient complained of neurological symptoms including poor balance, difficulty writing, shakiness, poor concentration, chronic headaches, insomnia, and fatigue. She also described remitting depression and anxiety. She stated that her health was “rapidly declining.”
Medical and family history
Her past medical history included malignant ventricular arrhythmia (MVA) in 2011 and long-standing insomnia and also included anxiety attacks, depression, migraines, hysterectomy, and root canal surgery, as well as a motor vehicle accident in the previous year. More recently, the patient had been diagnosed with arthritis, insomnia, and diverticulitis. Her family history included a mother with heart disease, osteoporosis, colon cancer, and anemia; no other family members had any chronic conditions. The patient’s medications included lorazepam, 3 mg bid; acetaminophen and oxycodone, 1 tablet of 7.5 mg/325 mg tablet qam; acetaminophen, 3 tablets bid; and butalbital, aspirin, caffeine combination 2 tablets bid.
Assessment
The patient’s biometric measures and vital signs were within normal range for body mass index (BMI), blood pressure, and heart rate at the initial visit (height 157 cm; weight 54.4 kg; BMI 21.9 kg/m2; systolic blood pressure 110 mmHg; diastolic blood pressure 65 mmHg; and heart rate 76 bpm). An organic acids urinary test was ordered, and the patient completed a baseline Health Appraisal Questionnaire. Additionally, the patient was asked to complete a total of 5 validated questionnaires to assess overall health and assessment of pain. The questionnaires included the Medical Symptoms Questionnaire, DN4 Questionnaire, Fibromyalgia Questionnaire, Brief Pain Inventory (Short Form), and Short-form McGill Pain Questionnaire (Figure 1). Preliminary functional diagnoses included altered microcirculation, neurogenic inflammation, hypoadrenalism, hypogonadism, gluten intolerance, hypomethylation, chronic insomnia, refractive macrocytosis, intestinal dysbiosis, intestinal hyperpermeability, interstitial acidosis, quinolinic acid toxicity, and impaired glutathione induction.
Therapy and outcome
On August 23, 2012, the patient started an oral program that consisted of an anti-inflammatory medical food to regulate the inflammatory cascade, as many of her symptoms were biological consequences of chronic inflammation. In addition, it included therapeutic levels of curcumin, ginger, hops, and Boswellia extracts, which have been shown to regulate multiple mechanisms of the inflammatory cascade including cyclo-oxygenase, lipoxygenase, protein kinase function, and cytokine biosynthesis.2-5 It also included a nutraceutical high in methylating nutrients, specifically S-adenosylmethionine (SAMe) and trimethylglycine, as well as a personalized botanical extract for microcirculation that contained Leonurus spp., rosemary, and capsicum, which act as circulatory stimulants and improve capillary flow and cardiac output.6-10 Lastly, it was recommended she take a glutathione-inducing nutraceutical that contained N-acetyl cysteine (NAC), niacin, broccoli sprout seed extract, selenium, and ascorbate. The patient was asked to change her food intake to plant-based foods to reduce arachidonic acid intake. She was started on weekly intravenous micronutrient therapy. This initial protocol was chosen to target the mechanisms and systems that directly and indirectly impact the neurogenic inflammation and facial pain. Working functional medicine diagnoses included neurogenic inflammation, impaired peripheral microcirculation, hypo-methylation, and insufficient glutathione induction.
At her next visit 2 months later (month 10, after her initial consult), the patient reported “feeling a lot better.” Her facial pain had diminished by approximately 30%, and she was able to discontinue her pain medications. She claimed that her energy and anxiety/depression levels had improved by an estimated 50% and 90%, respectfully. Her mental clarity increased, and she reported that she “had hope again.” After a review of the organic acid test with the patient, in addition to continuing her current regimen, she was also instructed to add the probiotic Saccharomyces boulardii to combat yeast identified in the test results to be producing endotoxic byproducts and leading to a systemic immune response and neural sensitization. The patient also reported dyspepsia and bloating daily.
The patient returned to the clinic for a follow-up visit after another 2 months (month 12), reporting further improvements in energy. She stated that her depression was resolved, but her sleep remained a problem. She reported an improvement of more than 60% in her facial nerve pain as compared to the baseline assessment. She no longer required pain medications but continued to require 3 mg of lorazepam on an as-needed basis for anxiety. Her gas and bloating was also reduced, but the patient did not quantify the change. The patient was instructed to start on a botanical hepatoprotectant that included silymarin, DHEA for the hypoadrenalism, magnesium malate to improve mitochondrial bioenergetics, and high-dose niacin (as nicotinic acid) and to begin to wean off of the lorazepam but to continue the infusion therapy as needed.
After 1 month (month 13), the patient returned to the clinic. While she was unsuccessful at discontinuing the lorazepam, she reported a 50% reduction in gas and bloating. In addition to continuing the DHEA, magnesium malate, and nicotinic acid, she was also prescribed a prokinetic botanical and functional fiber to reset transient time. The patient continued to improve over subsequent office visits (Figure 1).
Table 1 - Case Study 1: Laboratory Values
| Analyte (reference range) | May 2011 prior to therapy | May 2012 prior to therapy | August 2012 | October 2012 |
| Hemoglobin (g/L) (135-170) | 131 | 134 | | 128 |
| Hematocrit (0.38-0.490) | 0.39 | 0.41 | | 0.38 |
| RBC (x1012/L) (4.2-5.7) | 3.83 | 4.10 | | 3.84 |
| MCV (fL)a (80-97) | 101 | 99 | | 100 |
| MCH (pg) (27-32) | 34 | 33 | | 34 |
| MCHC (g/L) (320-360) | 341 | 332 | | 336 |
| RDW (%) (11.5-15.5) | 14 | 14.7 | | 14.5 |
| WBC (x109/L) (4-11) | 7 | 5.8 | | 6.6 |
| Platelets (x109/L) (145-400) | 234 | 226 | | 223 |
| MPV (fL) ((7.4-11.3) | 9.2 | 9.2 | | 9.3 |
| Neutrophils (x109/L) (1.8-7) | 4.83 | 3.60 | | 4.16 |
| Lymphocytes (x109/L) (1-3.2) | 1.54 | 1.51 | | 1.65 |
| Monocytes (x109/L) (0-0.8) | 0.42 | 0.52 | | 0.40 |
| Eosinophils (x109/L) (0-0.4) | 0.21 | 0.17 | | 0.33 |
| Basophils (x109/L) (0-0.2) | 0.00 | 0.17 | | 0.07 |
| Glucose (mmol/L) (3.3-6) | 5.1 | 4.9 | | |
| Insulin (pmol/L) (13-161) | 44 | 39 | | |
| HsCRP (mg/L) | 1.4 | 1.2 | | |
| Calcium (mmol/L) (2.15-2.6) | 2.35 | 2.35 | | |
| Magnesium (mmol/L) (0.65-1.05) | | 0.91 | | |
| Zinc (µmol/L) (8.7-19.1) | | 16 | | |
| Ferritin (µg/L) (25-200) | 93 | 113 | | |
| Vitamin B12 (pmol/L) (>133) | 806 | 805 | | |
| 25 Hydroxy vitamin D (nmol/L) (75-200) | 77 | 80 | | |
| ESR (mm/hour) | 4 | 5 | | |
| Total protein (g/L) (60-82) | 66 | | | |
| Albumin (g/L) (35-50) | 43 | 44 | | |
| Globulin (g/L) | 23 | | | |
| Creatine (µmol/L) (70-127) | 80 | 78 | | |
| eGFR (mL/min/1.73 m2) (60-89) | 62 | 63 | | |
| Alkaline phosphatase (U/L) | | 52 | | |
| GGT (U/L) (5-50) | | 32 | | 28 |
| AST (U/L) (6-42) | | 24 | | |
| ALT (U/L) (4-43) | 23 | | | |
| TSH (mU/L) (0.3-5.6) | 0.92 | 1.18 | | |
| LH (IU/L) (1.2-8.6) | 27 | | | |
| Cortisol (AM)(mmol/L) | 479 | 408 | | |
| DHEAS (µmol/L)b (0.05-7.8) | 1.3 | 1.2 | | 0.9 |
| Testosterone (nmol/L) (6-27) | <0.5 | <0.5 | | |
| Free testosterone (pmol/L) (15.6-146) | <0.5 | 0/6 | | |
| Prolactin (ng/mL) (2.6-13.1) | 8 | | | |
| Progesterone (ng/mL) (0.3-2.7) | 0.6 | <0.3 | | |
| Estradiol (pmol/L) (0-172) | <46 | <46 | | |
| Urate (µmol/L) (180-450) | 252 | 247 | | |
| ACTH (pmol/L) | <2 | | | |
| Estrone (pg/mL) (138-509) | 62 | 23 | | |
| IGF-1 (ng/mL) | 112 | | | |
| Urine pH (5-8) | | 5.5 | | |
| Urine Indican | +1 | +2 | | |
| Organic Acid Test:57 | | | | |
| Arabinose (fungal marker) | | | elevated | |
| DHPPA (beneficial bacterial marker) | | | elevated | |
| Oxalic acid | | | elevated | |
| Quinolinic acid/5HIAA | | | elevated | |
| Ascorbate | | | low | |
| Pyroglutamate (toxicity) | | | elevated | |
a Serum cobalamine levels were not measured in light of recent evidence that they may not reflect functional need.11
b DHEA levels did not increase with supplementation. The authors suggest this may be due to malabsorption or increase in utilization (stress induced) by the body.
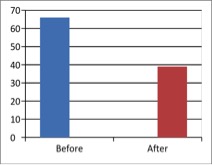
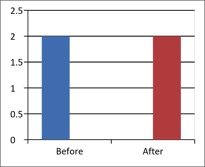
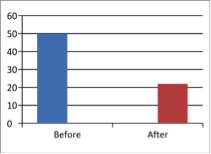
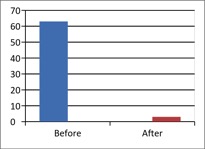
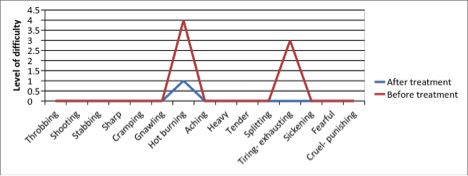
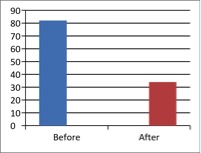
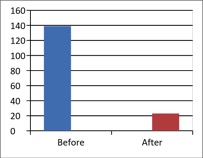
Figure 1 - Case Study 1: Assessment of Medical Symptoms and Pain at Prior to (Baseline) and After Therapy (Month 13, After 5 Months of Treatment)
a) Medical Symptoms Questionnaire (MSQ)
(Scale 0-100; Lower is Better)
b) DN4 Questionnaire
(Scale 0-10; Lower is Better)
c) Fibromyalgia Impact Questionnaire
(Scale 0-100; Lower is Better)
d) Brief Pain Inventory
(Scale 0-100; Lower is Better)
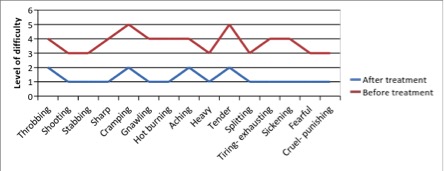
e) Short-form McGill Pain Questionnaire (0=none, 1=mild, 3=moderate, 4.5=severe)
Case Study 2: Chronic Thoracic Neuropathic Pain
A 42-year-old female presented to the clinic in October of 2010 complaining of worsening nerve pain and associated symptoms. Her nerve pain started after an epidural she received in conjunction with the birth of her second child. She described debilitating mid-back pain, at the site of the epidural, that radiated down her legs. Associated symptoms included difficulty concentrating, weight gain, excessive sweating, anxiety, inability to cope with stress, fatigue, poor sleep, and overall poor vitality.
Medical and family history
Her past medical history was unremarkable. Her current medical concerns included chronic neuropathic pain, hyperhydrosis, chronic migraines, sacroiliitis, metabolic syndrome, and insomnia. Her medication history included fentanyl, zolmitriptan, amitriptyline, atenolol, acetaminophen and oxycodone, zopiclone, vitamin D, glucosamine, omega-3, pregabalin, and gabapentin.
Assessment
The patient’s biometric measures were within normal range; however, vital signs indicated the patient was hypertensive (height 165 cm; weight 54.4 kg; BMI 19.1 kg/m2; systolic blood pressure 166 mmHg; diastolic blood pressure 99 mmHg). Visual inspection revealed mild swelling and peri-nasal and upper chest redness. The patient underwent an x-ray and a computed tomography (CT) scan. The results of the x-ray were normal, but her CT scan revealed sacroiliitis. The possible functional dysfunctions included hypomethylation, phase I and II detoxification biotransformation imbalance (a process wherein the p450 enzymes kinetics are out of synch with the conjugation of phase II leading to improper metabolite formation and poor detoxification capacity), sympathetic dominance (excess fight or flight response), hyperHPA (high cortisol production), impaired serotonin production, lymphatic congestion, dysinsulinemia, oxidative stress, hepatosis (nonspecific impaired liver function), gluten sensitivity, arachidonic acid toxicity, xenobiotic bioaccumulation, and suboptimal progesterone/testosterone concentrations.
Therapy and outcomes
After a medical diagnosis of neuropathic pain, the patient underwent a nerve block with her pain specialist that unfortunately did not improve her pain. She also tried onabotulinumtoxinA injection, which did not resolve the pain. In November 2010, the patient began a weekly intravenous micronutrient therapy with glutathione; a homeopathic formula for regulating pain transmission called Nerventropfen; and a botanical for regulating serotonin biosynthesis, synaptic release, and reuptake, which included a synergistic blend of St John’s Wort, Valerian root, and passiflora.11,12 She was advised to take a homeopathic detoxification kit to support liver, kidney, and lymphatic function and an insulin-signaling medical food called ultraglycemix to improve insulin sensitivity and intracellular signal transduction response.13-15 Finally, the patient was prescribed bioidentical testosterone and progesterone, as her labs were shown to be deficient. After 2 months of therapy, the patient reported being able to discontinue atenolol (as the pain was likely the cause for the hypertension) and amitriptyline and was able to reduce her reliance on a 100-µg/hour dose of the fentanyl patch, as she was able to change the patch less frequently. In addition to experiencing a reduction of hypersensitivities as the patient had symptoms of multiple chemical sensitivity (although it is unclear as to why), she also reported a 30% and 40% reduction in pain and anxiety, respectfully. Swelling and peri-nasal redness were reduced, and she had not had an episode of sweating. She was directed to stay on her current protocol, except for the infusion therapy. Upon her follow-up visit in April 2011 (month 6), the patient reported that she was able to reduce her fentanyl patch from 100 µg to 12 µg. On April 4, 2011, she was placed on a metabolic detoxification medical food that consisted of plant extracts and micronutrients shown to upregulate phase II detoxification pathways and that included magnesium sulphate, potassium citrate, rosemary, watercress, carotenoids, and epigallocatechin gallate.16 This was chosen as many environmental toxicants dysregulate pain transmission and induce an excitatory effect by excessive glutamate activity, even with ambient exposure.17
One month later (month 7), the patient reported having only 1 migraine in the previous 3 months, her sleep had improved, she had less swelling, and she had lost some weight. She also reported a 50% reduction in pain since the beginning of therapy. She was then instructed to begin a gluten-free (GF) diet as a result of the emerging research on gluten’s impact on the nervous system.18 After 30 days on the GF diet, she reported improved cognition and significantly more energy. Her bloating had resolved, and sleep was continuing to improve. In September 2011 (month 11), she was started on a hepatic biotransformation modulator to continue to stabilize phase I and II detoxification enzyme systems and continued her treatment with bioidentical progesterone and testosterone therapy. At that time, she was also started on a series of biopuncture sessions, a technique wherein low-dose plant extracts are injected into trigger points, and this continued to improve her pain. In March 2012 (month 17), she discontinued red meat on her own volition, and her pain decreased by another 50% from the previous visit. We had discussed the potential benefits of lowering arachidonic acid in the diet on a previous consult. In April 2012 (month 18), the patient was started on a formula designed to upregulate gene expression to induce the biosynthesis of glutathione, an NFkB-regulating nutraceutical to lessen the inflammatory cascade and sulforaphane, a phase II enzyme inducer. By January of 2013, 2 years since diagnosis (month 24) and initiation of therapy, the patient’s pain decreased by 80% compared to baseline, and her health and functionality continued to improve as demonstrated by improvement in pain questionnaire scores (Figure 2).
Table 2 - Case Study 2: Laboratory Values Before and After Diagnosis and Initiation of Therapy
| Analyte | September 2010 (prior to therapy) | April 2011 (6 months of therapy) | November 2011 (13 months of therapy) | April 2012 (18 months of therapy) |
| Hemoglobin (g/L) (120-160) | 150 | 139 | | 139 |
| Hematocrit (0.25-0.45) | 0.44 | 0.43 | | 0.41 |
| RBC (x1012/L) (4-5.1) | 4.89 | 4.65 | | 4.42 |
| MCV (fL) (80-100) | 89 | 91.6 | | 93 |
| MCH (pg) (27.5-33) | 31 | 29.9 | | 31 |
| MCHC (g/L) (305-360) | 343 | 326 | | 338 |
| RDW (%) (11.5-14.5) | 10.9 | 13.4 | | 13.5 |
| WBC (x109/L) (4-11) | 8 | 7.9 | | 8 |
| Platelets (x109/L) (150-400) | 378 | 330 | | 276 |
| MPV (fL) (7.4-11.3) | | | | 9.5 |
| Neutrophils (x109/L) (2-7.5) | 5.55 | 5.5 | | 5.36 |
| Lymphocytes (x109/L) (1-3.5) | 1.65 | 1.7 | | 1.68 |
| Monocytes (x109/L) (0.2-1) | 0.59 | 0.5 | | 0.56 |
| Eosinophils (x109/L) (0-0.5) | 0.16 | 0.3 | | 0.32 |
| Basophils (x109/L) (0-0.2) | 0.09 | 0.0 | | 0.08 |
| Glucose (mmol/L) 3.6-6) | 6.2 | 5.6 | | |
| Insulin (pmol/L) (13-161) | | 102 | | |
| Apo A (mg/dL) | 1.52 | | | |
| Apo B (g/L) | 1.29 | | | |
| APO B/A ratio | 0.85 | | | |
| Cholesterol (mmol/L) | 6.88 | 7.3 | 6.45 | |
| Triglyceride (mmol/L) | 1.21 | 1.34 | | |
| HDL (mmol/L) | 1.38 | 1.47 | | |
| LDL (mmol/L) | 4.94 | 5.22 | | |
| Total cholesterol/HDL ratio | 4.99 | 5 | | |
| Cholinesterase (U/L) | | | | 3365 |
| Fibrinogen (µmol/L) | | | | 3 |
| HsCRP (mg/L) | 0.58 | | | |
| Sodium (mmol/L) (135-147) | 141 | | | |
| Potassium (mmol/L) (3.5-5.5) | 4 | | | |
| Chloride (mmol/L) (100-110) | 103 | | | |
| Calcium (mmol/L) (2.15-2.6) | 2.45 | | | |
| Iron (13.5-17.5) | 13.4 | | | |
| Transferrin (g/L) | 3.2 | | | |
| Saturation (µmol/L) | 0.19 | | | |
| Ferritin (µg/L) (25-200) | 59 | | | |
| Vitamin B12 (pmol/L) (>133) | 216 | 411 | 344 | 339 |
| Folate, RBC (nmol/L) | 1600 | | | |
| 25 Hydroxy Vitamin D (nmol/L) (75-200) | 76 | | | |
| Albumin (g/L) (35-50) | 41 | | | 48 |
| Creatine (µmol/L) (60-127) | 72 | | | |
| eGFR (mL/min/1.73m2) (60-89) | 101 | | | |
| Urea (mmol/L) | 4.9 | | | |
| Creatine kinase (U/L) (60-127) | | | | 53 |
| Bilirubin total (µmol/LO) | 5 | | | |
| Alkaline (phosphatase (U/L) | 72 | | | |
| GGT (U/L) (5-50) | 28 | 25 | 20 | 16 |
| AST (U/L) (6-42) | 18 | | | |
| ALT (U/L) (4-43) | 18 | 28 | 14 | 14 |
| TSH (mU/L) (0.3-5.6) | 3.27 | 2.03 | 2.33 | 1.88 |
| Cortisol (AM)(mmol/L) | 540 | 294 | 396 | 316 |
| Cortisol (4 PM)(mmol/L) | 555 | 601 | | |
| DHEAS (µmol/L) (0.05-7.8) | | | 4.1 | |
| Free testosterone (pmol/L) (15.6-146) | 0.9 | 4.9 | | 2.1 |
| Progesterone (ng/mL) (0.3-2.7) | | 3 | 6.7 | 27.5 |
| 17-OH progesterone (nmol/L) | 1 | | | |
| Estradiol (pmol/L) (0-172) | | | 48 | |
| Urate (µmol/L) (180-450) | 285 | 214 | | |
| Estrone (pg/mL) (138-509) | | | 149 | |
| Estriol, unconjugated (nmol/L) (0-172) | | | | <0.25 |
When using a functional medicine, key systems such as inflammation, insulin, detoxification, stress response, and hormones are targeted. This approach is designed to shift a network of biological factors in a positive way, leading to symptom reduction and improved health. Key lab changes found in this patient include reduction in gamma-glutamyltransferase (GGT) levels, which suggests improved glutathione synthesis and may predict metabolic and vascular events as well as lowered cortisol as a result of a normalized HPA responses.19 The improvement in the HPA axis indirectly improved the thyroid axis and healthier levels of TSH due to communication between the HPA and HPT axis.20-22 Cholinesterase was also included as it has been used a marker of damage due to a toxic load.23 Testosterone levels also improved as a result of replacement therapy.
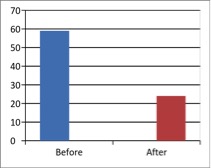
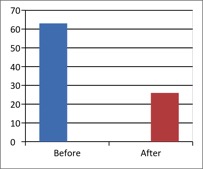
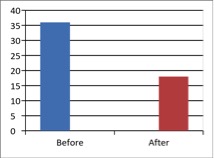
Figure 2 - Case Study 2: Assessment of Medical Symptoms and Pain at Prior to (Baseline) and After Therapy (Month 24)
a) Medical Symptoms Questionnaire (MSQ)
(Scale 0-100; Lower is Better)
b) DN4 Questionnaire
(Scale 0-10; Lower is Better)
c) Fibromyalgia Impact Questionnaire
(Scale 0-100; Lower is Better)
d) Brief Pain Inventory
(Scale 0-100; Lower is Better)
e) Short-form McGill Pain Questionnaire (0=none, 1=mild, 2=moderate, 3=severe)
Case Study 3: Paresthesia, Joint Pain, Fatigue, and an Inability to Cope With Stress
A 55-year-old female came to the clinic in January 2012 with long-standing (over 2 years), insidious onset and complaints including fatigue, inability to cope with stress, and poor stamina. Her primary concern at that time was fatigue, which had worsened significantly and steadily. Associated symptoms included daily anxiety, poor concentration and focus, and memory issues. She also explained that she had to eat every 2 hours or she felt faint and described tingling down her arms.
Medical and family history
The patient described a high level of recent stress and felt she had an inability to adapt and cope with it. She also complained of wakefulness, usually around 3:00 in the morning. She reported light-headedness every morning, which often reoccurred throughout the day. Her medication list included levothyroxine, vitamin D, and a probiotic. Social history was negative for alcohol or smoking.
Assessment
Physical exam showed the patient was positive for orthostatic hypotension. She completed a neurobehavioral questionnaire, which was positive for impaired cortisol/serotonin dynamics. She also completed an Identi-TTM stress questionnaire, the results of which were suggestive of a hyperadrenergic sympathetic tone, and the patient feeling “tired and wired.” Additionally, the patient was asked to complete the 5 validated questionnaires (Figure 3). Preliminary functional diagnoses included altered hypothalamic–pituitary–adrenal (HPA) dynamics, hyperadrenergic tone (dysautonomia), postprandial hypoglycemia, and generalized anxiety.
Therapy and outcomes
The patient was started on a series of intravenous micronutrient therapy (ascorbate, pyridoxine, dexpanthenol, magnesium sulfate, and B complex) for the purpose of supporting mitochondrial function and adrenal output, a peripherally acting adaptogenic formula that contained Glycerrhiza and Eleuthrococcus, DHEA to balance the cortisol:DHEA ratio, and passiflora extract as an anxiolytic.24 Her 1-month follow-up revealed a 75% improvement in daily energy, improved focus, and memory. She also reported that an improvement in the time it took to fall asleep at night. At this follow-up visit, the patient was instructed to discontinue passiflora and start Serenagen, a traditional Chinese medicine formula that consists of Rehmannia glutinosa, Angelica sinensis, Polygala tenufolia, Schizandra spp., Platycodon grandiflorum, and other synergistic botanicals that stabilize hemodynamic status and cardiac function and output during hyperadrenergic states.
At the patient’s second follow-up visit in May (month 5), she reported a very stable mood, even under stress. She also reported that the tingling in her extremities had temporarily resolved but had returned in the 2 weeks prior to the visit. She was instructed to discontinue the DHEA and start on a hops extract designed to selectively target NFkB and pro-inflammatory gene expression. She was also referred to a nutritionist for help with low-glycemic meal plans. Lab tests were performed (Table 3).
The patient returned to the clinic at month 9. Her tingling of extremities and her dizziness were resolved. She requested treatment for her sleeplessness and menopausal symptoms. In addition to continuing the hops extract, she was prescribed a phyto-hormone, Siberian rhubarb extract, and a phyto-progestogenic gonadotropic formula containing Bupleurum chinensis, Coleus forskohlii, and Dioscorea villosa to normalize hormone signaling through production, receptivity, and cellular response.
The patient returned for follow-up at 12 months and reported that she was sleeping better, had fewer problems falling asleep, and experienced less wakefulness. She had also stopped gluten, and her joint pain had reduced by 70%. She continued the hops extract and started an inflammatory-regulating analgesic botanical and a homeopathic formula. Salivary hormone testing that included a 4-point cortisol, DHEA, estrogen, progesterone and testosterone evaluation was performed. On her follow-up visit in January 2013 (month 12), she described a significant improvement in joint pain (Figure 1). Additionally, the results of the salivary testing performed revealed multiple hormone deficiencies (Table 3). In response, the patient was prescribed a hormone-balancing program that included progesterone, androgen, and testosterone-stimulating botanical support formulas. She continues to adhere to her treatment regimen for optimal health and is continuing to show improvement.
Table 3 - Case Study 3: Laboratory Blood Tests and Salivary Hormone Tests After 4 and 12 Months of Therapy, Respectively
Month 4: Blood test results| TSH (mU/L) | 4.1 7 |
| Free T4 (ng/L) | 8.9 |
| AM Cortisol (mmol/L) | 509 |
| Fasting insulin (pmol/L) | 28 |
| 2-hr post-prandial glucose (mmol/L) | 7.9 |
| Fasting glucose (mmol/L) | 5.3 |
Month 12: Salivary hormone results| Estrone (pg/mL) | 0.8 (low) |
| Estradiol (pmol/L) | 0.2 (low) |
| Estriol (nmol/L) | Normal |
| Progesterone (ng/mL) | <0.1 (low) |
| 17-OH Progesterone (nmol/L) | 6.4 (low) |
| DHEA (µmol/L) | 0.10 (low normal) |
| Testosterone (pmol/L) | 5 (low normal) |
| Cortisol curve (noon, evening, and night: mmol/L) | Low normal |
As a general comment in hormone therapy and monitoring, testing alone provides an incomplete picture of endocrine status. It is the opinion of the author that clinical signs and symptoms should be used to guide therapy, and regular objective testing should be used to ensure that levels are safely within range. Caution should be exhibited when considering adjusting hormone therapy based solely on lab markers.
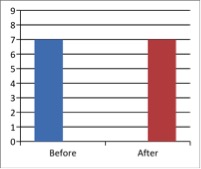
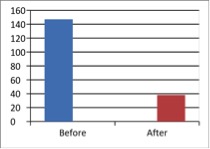
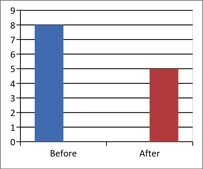
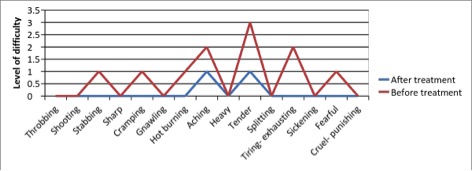
Figure 3 - Case Study 3: Assessment of Medical Symptoms and Pain at Prior to (Baseline) and After Therapy (Month 12)
a) Medical Symptoms Questionnaire (MSQ)
(Scale 0-100; Lower is Better)
b) DN4 Questionnaire
(Scale 0-10; Lower is Better)
c) Fibromyalgia Impact Questionnaire
(Scale 0-100; Lower is Better)
d) Brief Pain Inventory
(Scale 0-100; Lower is Better)
e) Short-form McGill Pain Questionnaire (0=none, 1=mild, 2=moderate, 3=severe)
Discussion
In each of the 3 case studies presented, patients received—in addition to a conventional diagnosis—a functional medicine approach to chronic pain. All patients showed a positive response by the end of the treatment period. Subjective outcome measures including the Short-form McGill Pain Questionnaire, DN4 Questionnaire, Medical Symptoms Questionnaire, Fibromyalgia Impact Questionnaire, and the Brief Pain Inventory scale improved from the time of diagnosis and start of therapy through the end of the follow-up period. Furthermore, no serious adverse events occurred over the course of the treatment periods, suggesting that subjects were adequately cared for and that all treatments were well tolerated by the patients.
The conventional model and the healthcare system are “largely built upon a reactive, sickness model, where care is focused in clinics, hospitals and other institutions.1 Yet chronic disease currently accounts for approximately 70% of the total deaths, and represents over 75% of direct healthcare costs in the United States.25,26 These statistics underscore the notion that the dominant challenges of modern day medicine have shifted away from acute care–related medical problems toward issues associated with chronic disease. As a result, the current medical model no longer meets the needs of the population and does not adequately address the increasing frequency of chronic, complex, multisystem diseases.
According to an article published in the Journal of the American Medical Association, “Chronic disease requires a practice of medicine quite different from that used for acute disease.”27 The functional medicine model represents a comprehensive approach to chronic disease whereby the focus is to understand the underlying dysfunction and create an individualized treatment program. The functional medicine model is more adequately suited to address the healthcare needs of today.
Functional medicine focuses on identifying and understanding the multiple pathological mechanisms known to be involved in complex, chronic disease. In this way, it is able to address core clinical imbalances that are collectively responsible for reducing the patient’s functionality and health and places a high emphasis on understanding underlying impairments that lead to dysfunction, rather than merely managing symptoms. This strategy also prioritizes antecedents (factors predisposing a patient to disease), triggers (factors provoking the symptoms), and mediators (biochemical factors leading to pathological change), allowing for the identification of unique pathological mechanisms.28 The functional medicine model engages both patient and health professional in a personalized, systems-oriented approach and uses emerging concepts in metabolomics, proteomics, and genomics with the best available evidence in nutritional, botanical, lifestyle, pharmaceutical, and hormonal interventions to identify underlying causes of disease of each individual patient and designs strategies to restore health and optimize therapeutic outcomes.29
Since networks pervade all aspects of human disease,30,31 any given diagnosable pain condition should not be considered an isolated clinical problem involving one biochemical pathway gone awry. Instead, pain syndromes can be thought to result from disturbances in a complex signaling network that may involve abnormal biochemical, social, and psychological domains, leading to altered sensation and transmission of pain signals.32
Recent research has further illustrated this concept by unraveling new connections that contribute to chronic pain syndromes. For example, it has been demonstrated that there is a relationship between the immune system and nerve pain.33 Researchers have found that peripheral blood neutrophils express neuropeptide nociceptin, which has been linked with pain signal transmission.33 Further, a connection exists between immune cells, neurons, and glia, suggesting that the restoration of altered signaling in these systems may represent a new treatment target.34 More recently, it has been established that matrix metalloproteinases play a role in the development of neuropathic pain associated with inflammatory cytokine production and microglial activation.35 Therefore, accumulating data suggest that triggers such as infection, ischemia, trauma, allergy, toxins, nutritional insufficiencies, and stress hormones may underlie many chronic pain syndromes. Suggesting that the site at which the disease or damage originated is important in identifying and understanding downstream effects felt as pain. Furthermore, a dysfunction in one system can lead to symptoms in another, and this dynamic, interconnected web-like interaction of various physiological processes may be interpreted as pain signal; pain may be a metaphor for dysfunction.32,36
Conclusion
The pain response involves individual and complex signaling pathways and may also involve other undetected underlying dysfunctions. The case series presented here highlights the importance of using the functional medicine model. Understanding the various dysfunctional processes unique to each patient and applying the best level of evidence to restore these fundamental processes is critical in improving patient outcomes and helping patients make informed decisions. Furthermore, in order to implement functional medicine, clinicians must be critical in their thinking and decisions to provide the depth of care necessary to improve patients’ overall health and functionality. The physician-patient experience needs to be a collaborative partnership for success. As illustrated in the above cases, patients were educated with respect to triggers, antecedents, and mediators that led to their poor health and unique story. In addition, they were offered various treatment options to consider. Once educated, the patients made informed health choices based on belief system, backgrounds, and experiences as to how they wanted to be treated and what outcomes we could expect. Clinical research using randomized controlled trials in specific populations (eg, neuropathic pain) is warranted to determine if the functional medicine model is superior to conventional treatment.