Reference
Wallen ZD, Appah M, Dean MN, et al. Characterizing dysbiosis of gut microbiome in PD: evidence for overabundance of opportunistic pathogens. NPJ Parkinsons Dis. 2020;6:11.
Design
A case-control study of gut microbiome–wide associations of people with Parkinson disease (PD) compared to neuro-healthy controls.
Participants
Two data sets were presented in the study. Data set 1 included 197 Parkinson cases and 130 neuro-healthy controls with participants from Albany, New York, Seattle, Washington, and Atlanta, Georgia. Data set 2 included 323 Parkinson cases and 184 neuro-healthy controls from Birmingham, Alabama.
Primary Outcome Measures
The primary outcome for this study was abundance of gastrointestinal microbes and patterns of co-occurring microbial genera.
Researchers analyzed all data with and without confounding factors including geography, sex, age, constipation in the last 3 months, gastrointestinal discomfort, daily fruit and vegetable consumption, body mass index (BMI), alcohol consumption, weight loss, and PD medication.
Key Findings
Researchers discovered 3 clusters that encompassed 15 distinct genera in people with PD but not neuro-healthy controls, suggesting these microbes are associated with PD.
Cluster 1: Those with PD had an abundance of Porphyromonas, Prevotella, and Corynebacterium_1 genera compared to controls. Although these microbes are commensal in normal numbers, when overgrown in the gut, they can contribute to disease progression. The authors suggest that the microbes elevated in PD could be acting as opportunistic pathogens.
Cluster 2 contained 10 genera that were in lesser abundance in the 2 cohorts of participants with PD compared to controls. The majority of the genera in cluster 2 were anaerobic, Gram-positive bacteria in the Ruminococcaceae and Lachnospiraceae families, which are well known to produce butyrate as well as other short-chain fatty acids in the gut.
Cluster 3 is the most curious. Although those with PD were not taking any probiotic supplementation, they had higher abundance of Lactobacillus and Bifidobacteria spp.
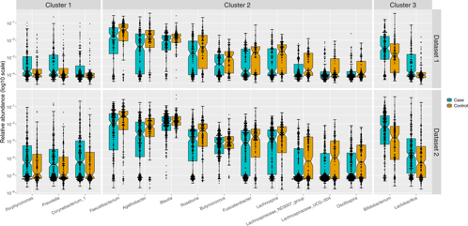
"Relative abundances in PD cases (blue) and controls (orange) were plotted as log10 scale on the y-axis. Sample size was 201 cases and 132 controls in dataset 1, and 323 cases and 184 controls in dataset 2. Each dot represents a sample, plotted according to the relative abundance of the genus in the sample. The notch in each box indicates the confidence interval of the median. The bottom, middle, and top boundaries of each box represent the first, second (median), and third quartiles of the relative abundances. The whiskers (lines extending from the top and bottom of the box and ending in horizontal cap) extend to points within 1.5 times the interquartile range. The points extending above the whiskers are outliers.” Reprinted under Creative Commons 4.0 license.
Practice Implications
Gut dysbiosis is increasingly evident in Parkinson disease.1-3 While it’s tempting to focus on the brain in a neurological disease, it’s clear that the gut is affecting disease progression.1 Clinically, this study suggests that practitioners should be addressing the gut along with the brain in their patients with PD.
What inhabits the gut and forms the microbiome is influenced mostly by diet. Prebiotics, such as those found in fruits, vegetables, mushrooms, and legumes, lead to increased production of butyrate, an anti-inflammatory short-chain fatty acid (SFCA). Gut microbes ferment nondigestible oligosaccharides, including onion, leeks, asparagus, artichokes, and beets, to create butyrate.4 Sugar alcohols, such as polyols from fruit,5 and some proteins can also support butyrate production. However, endogenous butyrate production relies on the proper microbial species to ferment the fibers, and this study showed that these SCFA-producing microbes are deficient in people with PD (cluster 2).
One strategy for increasing butyrate involves increasing the quantity of fruits and vegetables in the diets of people with PD. Indeed, research suggests that a Mediterranean diet can reduce risk or delay disease onset for PD.6-9 Separately, a ketogenic diet can increase hydroxy-butyrate, and 2 small studies suggest that some people with PD (but not all) respond well to a ketogenic diet.10,11 It’s also possible that some people with PD will need supplemental butyrate due to the low number of SCFA-producing bacteria colonizing their colon; however, this remains speculative as there are no trials confirming this presumption.
The increased numbers of Bifidobacterium and Lactobacillus suggest that administration of these particular probiotics is not indicated in people with PD and may, in fact, influence the amount of levodopa medication needed for symptom control.
Another strategy for increasing butyrate is to prescribe probiotics containing butyrate-producing microbes. However, over-the-counter probiotics more often than not contain Lactobacillus spp and Bifidobacterium spp, both of which were higher in abundance in those with PD in the current study under review. Normally, their presence is associated with a more favorable environment for the butyrate-producing bacteria to thrive. The authors contend that high abundance of these popular probiotics may be due to the use of PD medications, including levodopa. In fact, Lactobacillus spp metabolize levodopa into dopamine, so it may be considered an energy substrate of that genera. The more levodopa/carbidopa that a person takes, the more Lactobacillus spp grow to metabolize it, which then requires administration of higher and higher amounts of levodopa/carbidopa. Ingestion of plants and dairy can also increase Lactobacillus and Bifidobacterium spp. Alternatively, the Lactobacillus and Bifidobacterium could be compensatory, where the gut is increasing their abundance in an attempt to counter the low SCFA-producing species exhibited in the gut of those with PD.
The increased numbers of Bifidobacterium and Lactobacillus suggest that administration of these particular probiotics is not indicated in people with PD and may, in fact, influence the amount of levodopa medication needed for symptom control. However, there is 1 past study that demonstrates that probiotics (in fermented milk) can reduce constipation in people with PD12—perhaps because increased Lactobacillus increases dopamine, which is responsible for gut contraction. Regardless, administration of probiotics must be carefully considered in these patients.
Several recent research studies have demonstrated the impact of nonantibiotic pharmaceuticals on the microbiome.13,14 This study confirms those results in PD. While PD medications did not affect the overgrowth of pathogenic microbes, these medications may have decreased SCFA-producing microbes and increased the Bifidobacterium and Lactobacillus spp. As clinicians, it’s important to recognize that all pharmaceuticals and herbs will have microbiome relationships to consider. As we learn how dysbiosis is involved in PD, clinicians should be ready to modify what may be long-held beliefs about what is useful or possibly harmful in their approach to dysbiosis in this population.






