Abstract
Cancer cachexia is a multifactorial syndrome characterized by loss of lean body mass, which may adversely affect a patient’s overall survival, quality of life, level of physical activity, and ability to receive antineoplastic therapy.1 Where full eradication of tumor burden is not achieved, multimodal prevention or treatment of cachexia is indicated. This article serves to highlight strategies to consider based on available evidence and treatment goals in cancer cachexia.
Introduction
Cachexia is a condition secondary to a primary disease process characterized by wasting, weight loss, muscle atrophy, fatigue, weakness, and loss of appetite in patients not actively trying to lose weight or making behavioral or lifestyle changes. It is estimated to be the cause of death in more than 30% of cancer patients, and more than half of all cancer patients have cachexia at the time of death.2 In advanced cancer, the prevalence of cachexia is thought to be between 60% and 80%.2
Precise percentages of cachexia prevalence are, however, difficult to capture due to the use of different criteria to define cachexia by the various reporting groups. Regardless, there is an urgent need to employ supportive care strategies that may effectively work to prevent or slow the development of cachexia and thereby improve treatment tolerance, response to treatment, quality of life, and overall survival.3
Classification of Cachexia Stages
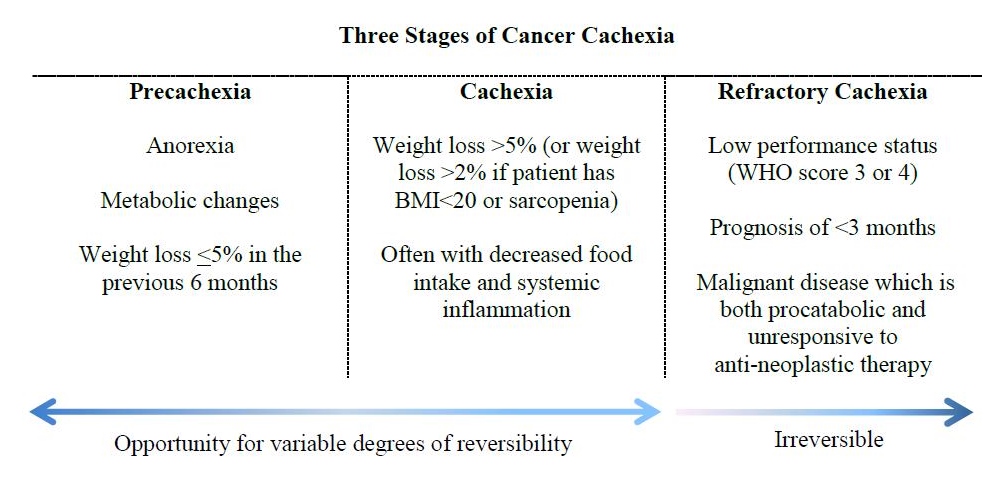
A diagnostic framework developed in 2011 has been proposed that defines cancer cachexia as occurring along a continuum consisting of 3 stages: precachexia, cachexia, and refractory cachexia (Figure 1).4 The precachexia stage is classified as including anorexia, metabolic changes, and weight loss ≤5% in the previous 6 months.4 The cachexia stage is classified as involving weight loss >5% (or weight loss >2% if the patient has BMI<20 kg/m2 or sarcopenia) and often with decreased food intake and systemic inflammation.4 The refractory cachexia stage is classified as involving low performance status (ECOG score 3 or 4), prognosis of <3 months, and malignant disease that is both procatabolic and unresponsive to antineoplastic therapy.4 Proposed refinements have been made to the criteria of the precachexia stage, yet challenges persist in defining its parameters in a way that makes it a clinically relevant tool.5 The concept of precachexia, however, is important to grasp in that this is the stage at which metabolic changes have already begun to take place and progression to cachexia may be prevented by initiation of early interventions. This initial period of the cancer cachexia trajectory tends to be barely perceptible clinically and thus can be easily missed. Reversible risk factors should be addressed as early as possible on the continuum and must continue to be addressed through the cachexia stages. Identification of refractory cachexia is useful in determining when ethical considerations should be evaluated in the goals of naturopathic supportive care, and a shift to strictly palliative support should be discussed at that time.
Figure 1. The stages of cancer cachexia
Risk Factors, Pathophysiology, and Diagnostics
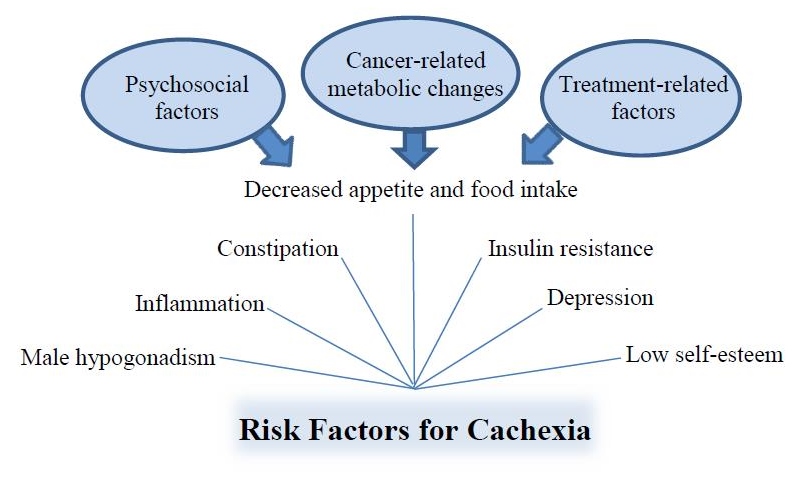
There are several risk factors for cancer cachexia, most of which are reversible if caught early enough (Figure 2). Risk factors include constipation, depression, inflammation, insulin resistance, male hypogonadism, low self-esteem, decreased appetite, and decreased food intake.1 In cancer patients, decreased appetite may be due to standard antineoplastic treatment–related toxicities such as dysgeusia, nausea and vomiting, odynophagia from mucositis, and early satiety after major abdominal surgical procedures. Psychosocial factors such as depression or increased stress also may contribute to decreased appetite. Cancer-related metabolic changes are considered a primary driving force behind decreases in appetite not due to treatment or psychosocial-related factors, which, along with loss of lean body mass, characterize the catabolic process associated with cachexia.1
Figure 2. Risk factors for cancer cachexia
Cancer-related metabolic changes include increases in muscle catabolism, resting energy expenditure, metabolism, cortisol, inflammatory cytokine production, and insulin resistance. While the governing process to these systemic catabolic changes remains unknown, recent evidence implicates a central nervous system role—namely chronic hypothalamic inflammation—as a possible source.6
Cachexia is characterized by lean muscle loss as the body shifts from an anabolic state to a catabolic state, progressive fatigue and weakness, and a decline in performance status. As fatigue and anorexia may manifest in cancer patients for various reasons, it is weight loss with decreased muscle strength that can help identify cachexia.1 Dynamometry or muscle strength testing may be employed for this assessment. However, more often, gross evaluation of strength is assessed through resistive force testing of the arms and upper legs by a clinician.
Diagnostic lab markers may be used to identify the magnitude of cancer-related metabolic changes. These might include but are not limited to C-reactive protein (CRP), serum albumin, hemoglobin, TNF-alpha, IL-6, ghrelin, and leptin.1,7-10 Although no formal validated guidelines currently exist on specific parameters to diagnose cachexia, in 2008 it was proposed that any elevation seen in CRP or IL-6 or any decrease in hemoglobin or serum albumin might be used in combination with other parameters such as anorexia, fatigue, decreased muscle strength, and loss of lean body mass to arrive at a diagnosis of cachexia.9 A study of 136 male cancer patients in 2012 found that elevation of TNF-alpha and IL-6 independently predicted survival beyond cancer stage alone; however, the researchers found that low serum albumin alongside weight loss made use of parameters that were more readily available and that offered similar ability in predicting survival as compared to inflammatory markers.7 A 2014 study evaluated the utility of ghrelin and leptin in diagnosing cachexia and predicting survival. Researchers found that ghrelin levels above 663 ng/mL (sensitivity 83%; specificity 98%) and leptin levels below 31 ng/mL (sensitivity 79%; specificity 73%) were superior to the use of low albumin (sensitivity 63%; specificity 69.4%) in diagnosing cachexia and predicting survival.11 While laboratory biomarkers may be promising in the context of identifying the presence or magnitude of cachexia, values also may be abnormal due to other factors, and cachexia can sometimes be present without abnormalities in these markers. Their use therefore may have limited clinical value.
A 2015 comprehensive review of 27 clinical trials on bioelectrical impedance analysis (BIA)/phase angle found that BIA/phase angle measurements “can benefit in the clinical management of cancer patients in multiple ways: in the prevention; diagnosis; prognosis; and outcomes related to treatments that affect nutritional and overall health status.”10 BIA measures resistance and reactance of electric current in body tissues. From these values, measures of total body water, fat-free mass, and fat mass can be derived. Phase angle is a measure derived directly from the resistance and reactance values and serves as an independent prognostic variable. A low phase angle correlates with increased malnutrition status, increased severity of disease, and lower overall survival. BIA measurements are of greatest utility when obtained at baseline and repeated throughout an individual’s course of treatment, measuring changes in an individual over time. In this way, the efficacy of management and predictive value of the longitudinal changes can be better determined so that supportive care strategies can be modified accordingly and aggressiveness of nutritional interventions increased or decreased as needed.10
Treatment of Cachexia
There is no better way to treat and reverse cachexia than to decrease the overall tumor burden, but in cases in which full eradication of tumor burden is not achieved, multimodal prevention or treatment of cachexia is indicated. Conventional methods beyond treating the cancer itself include pharmaceutical agents such as appetite stimulants (eg, megastrol acetate, dronabinol), steroids, testosterone, or nonsteroidal anti-inflammatory drugs. Studies have shown that a combination of integrative therapies has significantly improved outcomes in the treatment of cancer cachexia vs use of a single therapy alone.12-16 There is a consensus among experts that multimodal treatment (medication, nutrition, and other non-pharmaceutical strategies) to address the various contributing factors to cachexia should be offered to cancer patients in the precachexia and cachexia stages.17,18
A thoughtful approach should be taken in encouraging food choices that will both meet the protein and caloric intake goals and prevent worsening of other host conditions that contribute to cachexia progression.
Nutritional counseling in cachexia typically focuses on increasing protein and caloric intake. Based on this nutritional approach as a single modality, evidence strongly suggests that early intervention in precachexia prior to CRP elevation can be effective in preventing or slowing weight loss.1,4,18 Evidence to suggest there is benefit of increased protein and caloric intake on physical function or quality of life once in the refractory cachexia stage is insufficient; however, nutritional counseling in this stage may be beneficial in helping the patient and caregivers understand the changes occurring and the limitations of nutrition during this time.18 Ultimately, once the systemic inflammatory response has begun, increased protein and caloric intake alone are inadequate to maintain weight as the inflammatory response dampens anabolism.1 In consideration of the implications of systemic inflammation along the cachexia continuum and the inability of increased protein and caloric intake alone to address the catabolic process, it is advised to continue encouraging anti-inflammatory food choices that also reduce oxidative stress and insulin resistance while providing essential nutrients even in the palliative care setting.19 A thoughtful approach should be taken in encouraging food choices that will both meet the protein and caloric intake goals and prevent worsening of other host conditions that contribute to cachexia progression. Although dietary approaches such as ketogenic diets and fasting are sometimes recommended for cancer patients, human clinical trial data on the effects of these specific strategies on the progression of cachexia in precachectic or cachectic cancer patients are not available at the time of this writing. However, consistent restriction (vs intermittent restriction) of calories or protein is suspected to worsen cachexia and is thus not advised for this patient population.
Exercise may attenuate the catabolic process of cachexia through several mechanisms, including decreasing insulin resistance, reducing inflammation, and modulating the breakdown of muscle mass.20 Though clinical trials of exercise therapy in defined cachectic cancer patient populations are lacking, a Cochrane review concluded there is strong rationale for the use of exercise in cancer cachexia.21 Multiple reviews and opinion articles have been published in support of exercise therapy as early intervention or prevention, as well as throughout more pronounced cachectic states.20,22-27 Both aerobic and resistance training have been shown to be beneficial in preclinical studies, although through different mechanisms. Aerobic activity lends more to attenuation of insulin resistance and inflammation, whereas resistance training exerts positive effects on lean muscle mass and strength.22-25 Arguments could be made for the encouragement of either form, or a combination of the 2, in the cachexia setting. Any specific recommendations should be made with consideration for the individual’s physical limitations, bone health and fracture risk, comorbidities, and preferences. Exercise tolerance may be decreased in patients with significant fatigue, anemia, or cardiac dysfunction. Care should be taken in tailoring exercise recommendations for these patients so as not to exacerbate these conditions.23
Non-pharmaceutical agents can be used in a multimodal approach that aims at preventing or treating cachexia. Often times, multipurpose supplemental agents such as those discussed in the following paragraphs will also prove useful for improving response to and tolerance of cancer treatments and can be continued throughout the long-term course of care. Use of nutritional, botanical, or vitamin supplements should include consideration of any potential interactions with antineoplastic therapies or medications, and use should be considered on an individual patient basis. Disease-specific and treatment-specific options should be considered in choosing targeted supplement support. For example, use of pancreatic enzymes may be a consideration in the setting of exocrine pancreatic insufficiency secondary to a pancreatic tumor or following resection of the involved malignant tissues. A standard naturopathic approach should also be taken in addressing an individual’s set of risk factors. The following is a look at some agents that may have a role in addressing some of the metabolic changes occurring in cachexia:
- Amine supplementation may decrease proteolysis and increase protein synthesis in skeletal muscle.28 Creatine is an amine thought to improve skeletal muscle mass and function.29 It was administered in a double-blind, randomized, controlled trial that evaluated its effects on nutritional status, quality of life, and muscle function in 16 colorectal cancer patients. Participants took 5 g twice daily for the first week, followed by 2.5 g twice daily for 7 weeks. Results showed a significant improvement in phase angle after 8 weeks of creatine supplementation.30 Branched chain amino acids (BCAAs), consisting of leucine, isoleucine, and valine, have also been shown to be useful energy sources, protein synthesis stimulators, and anti-inflammatory agents.31-33 Small human trials suggest improvement in outcomes such as increased oral intake and increased protein metabolism and synthesis with the use of BCAAs in cancer patients.34-36
- L-carnitine deficiency is thought to contribute to cancer cachexia progression.37 L-carnitine has been used in 2 randomized phase III clinical trials evaluating multimodal treatment in cancer cachexia, both of which showed improved outcomes in the combination-therapy arms.12,13 A placebo-controlled, randomized, double-blind multicenter trial evaluated the use of oral L-carnitine 4 g daily taken for 12 weeks by 26 advanced and irresectable pancreatic cancer patients. At the time of study enrollment, 88% of patients in the placebo group and 92% of patients in the L-carnitine group were receiving chemotherapy. Results showed statistically significant increased body mass index in the treatment arm vs a decrease in the control arm. A trend toward improved nutritional status and quality of life was also seen in the treatment arm.38
- Omega-3 fatty acid supplementation has been widely reviewed for extensive clinical trial evidence supporting its utility in improving clinical outcomes in cancer.39-41 Reviews consistently suggest that increased quality of life, increased physical activity, improved response to chemotherapy, and decreased inflammation are among the benefits of omega-3 fatty acid supplementation in cancer patients. In the latest review, authors concluded that omega-3 fatty acids, when used as part of a multimodal strategy, are effective in cachexia.41 In a randomized, controlled, crossover trial of 20 healthy subjects to evaluate the effects on appetite of 3 g EPA+DHA taken daily for 3 weeks, results were significant for less postprandial sensation of fullness in both genders and, in women, a significant increase in desire to eat with use of fish oil supplementation.42 A randomized controlled trial (N=68) comparing the daily use of a standard protein- and energy-dense nutritional formula to a comparable one enriched with omega-3 fatty acids (7.24 g daily), probiotics, and additional micronutrients showed a statistically significant (P<0.05) increase in body weight in cachectic head and neck cancer patients undergoing an 8-week course of radiation treatment when the omega-3 fatty acid–enriched formula was used during and 1 month after their radiation course vs the standard formula.43 A single-arm phase II study evaluating the use of combination therapy taken daily for 4 months consisting of antioxidants, a nutritional formula enriched with omega-3 fatty acids (3.12 g EPA+DHA per day), medroxyprogesterone acetate, and celecoxib showed significant improvement in body weight, lean body mass and appetite in the 39 patients with advanced cancer who presented at baseline with cancer-related anorexia/cachexia.14 In a phase III randomized study, 332 patients with cancer-related anorexia/cachexia were assigned to 1 of 5 treatment arms: arm 1, medroxyprogesterone or megestrol acetate; arm 2, oral EPA-enriched nutritional formula (2.2 g of EPA daily); arm 3, L-carnitine; arm 4, thalidomide; arm 5, a combination of therapies used in arms 1-4. Treatment was of 4 months’ duration, and results showed superiority of arm 5 over the other treatment arms, with a significant increase in lean body mass, decrease in resting energy expenditure, improvement in fatigue, increase in appetite, decrease in IL-6 and TNF-alpha, and improvement in performance status.12
- Melatonin has been shown in clinical trials to be beneficial for cancer patients receiving chemotherapy, radiation, supportive care, or palliative care and to possibly increase overall survival when used as part of a multimodal strategy. The research suggests melatonin has antioxidant, antiproliferative, and immune-modulating effects and also decreases the intensity of chemotherapy-induced side effects.44 In regards to cachexia specifically, results of a small clinical trial evaluating melatonin in combination with fish oil showed that the combination had a weight-stabilizing effect in cachectic patients.45 Results of another clinical trial evaluating melatonin in combination with supportive care vs supportive care alone in patients with metastatic solid tumor showed decreased rate of weight loss in the combination arm.46 A randomized, double-blind, placebo-controlled study evaluating melatonin as a single therapy for cachexia was conducted in patients with advanced cancer but terminated after only 28 days due to lack of significant differences seen between treatment arms in weight, appetite, and quality of life parameters.47 However, the authors note that 32% of their patients did not complete the trial due to advanced disease and further suggest that melatonin may have had a more significant role if not initiated so late in the disease trajectory.
Other supplements that may be worth consideration as part of a multimodal approach in the prevention or treatment of cachexia include vitamin D,19,48 magnesium,49 antioxidant therapies,50 and anti-inflammatory agents such as curcumin,19 quercetin,51 and boswellia.19 These may further address some of the underlying metabolic changes in cancer cachexia; however, they have been less researched for this specific purpose in cachectic patient populations.
Conclusion
Navigating the complex terrain of cancer cachexia can be done strategically and may improve treatment tolerance, response to treatment, quality of life, and overall survival. As always, physicians must take into consideration all factors relating to the individual patient including but not limited to diagnosis, prognosis, involved sites of disease, symptoms, physical limitations, treatment and related side effects, psychosocial circumstances, socioeconomic factors, cultural and religious backgrounds, and patient preferences. Identifying early on a patient’s risk factors for cachexia and addressing them then may slow or reverse the progression of refractory cachexia. Taking action on nutritional, exercise, or supplement recommendations should always be patient-driven, and caregivers should be counseled accordingly. Understanding and communicating the limitations of naturopathic support therapies when they are initiated late in the course of the cachexia trajectory can reduce unrealistic expectations and decrease burdens on patients and caregivers while educating them on the changes that are occurring.52,53 While ongoing and future clinical trials on multimodal treatments for cancer cachexia are much needed and may help us to achieve improvements in outcomes such as quality of life and overall survival for our patients, the synthesis of currently available evidence this article provides serves as a basis and call for routinely employing an integrated approach in an effort to diminish the suffering experienced by those who are at risk for or affected by cancer cachexia.





